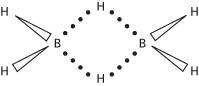
Any of a group of compounds of boron and hydrogen, many of which can be prepared by the action of acid on magnesium boride (MgB2). Others are made by pyrolysis of the products of this reaction in the presence of hydrogen and other reagents. They are all volatile, reactive, and oxidize readily in air, some explosively so. The boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model (see electron-deficient compound). The simplest example is diborane (B2H6): see formula. Other boranes include B4H10, B5H9, B5H11, B6H10, and B10H4. The larger borane molecules have open or closed polyhedra of boron atoms. In addition, there is a wide range of borane derivatives containing atoms of other elements, such as carbon and phosphorus. Borohydride ions of the type B6H62- also exist. Boranes and borohydride ions are classified according to their structure. Those with a complete polyhedron are said to have a closo-structure. Those in which the polyhedron is incomplete by loss of one vertex have a nido-structure (from the Greek for ‘nest’). Those with open structures by removal of two or more vertices have an arachno structure (from the Greek for ’spider’). See also Wade’s rules.
Borane
- dongle
- Donnan equilibrium
- Donoho, David Leigh (1957)
- donor
- do-nothing instruction
- Don Pacifico affair
- don’t care
- don’t feed the troll
- Doob, Joseph Leo (1910–2004)
- doomsday argument
- dopa
- dopamine
- dope vector
- doping
- doping compensation
- doping level
- doping profile
- Doppler broadening
- Doppler, Christian Johann
- Doppler, Christian Johann (1803–53)
- Doppler cooling
- Doppler effect
- dopplergram
- Doppler Orbitography and Radiopositioning Integrated by Satellite
- Doppler orbitography and radiopositioning integrated by satellite