An accumulator in which the electrodes are made of lead and the electrolyte consists of dilute sulphuric acid. The electrodes are usually cast from a lead alloy containing 7–12% of antimony (to give increased hardness and corrosion resistance) and a small amount of tin (for better casting properties). The electrodes are coated with a paste of lead(II) oxide (PbO) and finely divided lead; after insertion into the electrolyte a ‘forming’ current is passed through the cell to convert the PbO on the negative plate into a sponge of finely divided lead. On the positive plate the PbO is converted to lead(IV) oxide (PbO2). The equation for the overall reaction during discharge is:
The reaction is reversed during charging. Each cell gives an e.m.f. of about 2 volts and in motor vehicles a 12-volt battery of six cells is usually used. The lead-acid battery produces 80–120 kJ per kilogram. Compare nickel–iron accumulator.
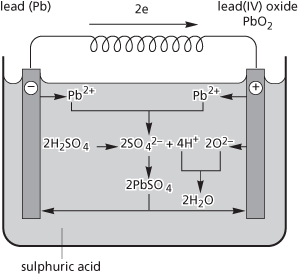
Lead-acid accumulator
- curvature of field
- curvature of space
- curvature of spacetime
- curvature radiation
- curve
- curve compression
- curve-fitting problem
- curve of growth
- curve sketching
- curvilinear coordinates
- curvilinear regression model
- curvilinear relation
- Curvolithus
- Curzon, George Nathaniel, 1st Marquis Curzon of Kedleston (1859–1925)
- Cush
- cusp
- cuspate foreland
- cusp cap
- cuspidate
- Custer, George (1839–76)
- custom
- customary units
- customer bounty
- customer data integration
- customer relationship management