The minimum energy required for a chemical reaction to take place. In a reaction, the reactant molecules come together and chemical bonds are stretched, broken, and formed in producing the products. During this process the energy of the system increases to a maximum, then decreases to the energy of the products (see illustration). The activation energy is the difference between the maximum energy and the energy of the reactants; i.e. it is the energy barrier that has to be overcome for the reaction to proceed. The activation energy determines the way in which the rate of the reaction varies with temperature (see Arrhenius equation). It is usual to express activation energies in joules per mole of reactants. An activation energy greater than 200 KJ mol−1 suggests that a bond has been completely broken in forming the transition state (as in the SN1 reaction). A lower figure suggests incomplete breakage (as in the SN2 reaction). See also activated-complex theory.
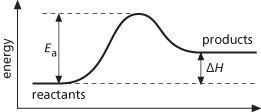
Activation energy
The minimum energy required to activate one mole of a substance to cause a chemical reaction to take place. For a chemical reaction to proceed, the reactants are converted to products in which the energy increases to a maximum and falls to the energy of the products (see Fig. 1). The activation energy is the difference between the maximum energy and the energy of the reactants and is therefore the energy than needs to be overcome. See arrhenius equation.
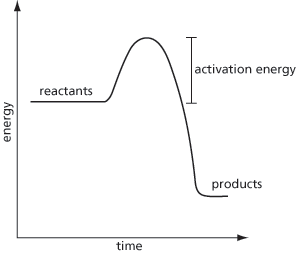
Fig. 1
Symbol Ea. The minimum energy required for a chemical reaction to take place. In a reaction, the reactant molecules come together and chemical bonds are stretched, broken, and formed in producing the products. During this process the energy of the system increases to a maximum, then decreases to the energy of the products. The activation energy is the difference between the maximum energy and the energy of the reactants; i.e. it is the energy barrier that has to be overcome for the reaction to proceed. The activation energy determines the way in which the rate of the reaction varies with temperature. It is usual to express activation energies in joules per mole of reactants.
The energy that must be delivered to a system in order to increase the incidence within it of reactive molecules, thus initiating a reaction.
- schedule number
- scheduler
- scheduling
- scheduling algorithm
- Scheele, Karl Wilhelm
- Scheele, Karl Wilhelm (1742–86)
- scheelite
- Scheer, Reinhard (1863–1928)
- Scheffé, Henry (1907–77)
- Scheffé test
- Scheiner, Christoph (1573–1650)
- Scheinwoodian
- Scheler, Max Ferdinand (1874–1928)
- Schelling, Friedrich Wilhelm Joseph von (1775–1854)
- schema
- schematic
- schematic flow diagram
- SCHEME
- Schengen Area
- Schering bridge
- Scheuchzer, Johann Jacob (1672–1733)
- Schiaparelli
- Schiaparelli, Giovanni Virginio
- Schiaparelli, Giovanni Virginio (1835–1910)
- schiefspiegler telescope