A solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components. The minimum freezing point for a set of components is called the eutectic point. Low melting-point alloys are usually eutectic mixtures.
A solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components. The minimum freezing point for a set of components is called the eutectic point. Low-melting-point alloys are usually eutectic mixtures.
A mixture of substances having the lowest freezing point of all mixtures of the substances. The eutectic point is the lowest temperature at which a solution can exist and that three condensed phases can co-exist (see Fig. 20). Low melting-point alloys are usually eutectic mixtures. For example, the freezing point of pure lead and pure tin are 327°C and 232°C, respectively, whereas a mixture with 62 per cent lead (soft solder) has a eutectic point of 183°C.
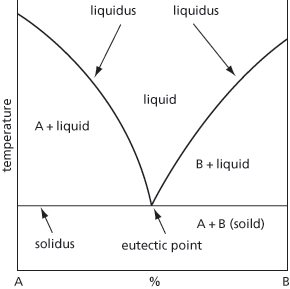
Fig. 20 Eutectic point
A mixture of elements that retains its composition in both solid and liquid form: there is no segregation of the components. Eutectic mixtures are often used to form electrical contacts, usually ohmic contacts, to semiconductor devices. For example, a gold-germanium eutectic mixture (88:12 w/o Au:Ge) is used as the basis of ohmic contacts to n-type gallium arsenide, where the gold provides the metallic contact and the germanium dopes heavily the surface of the gallium arsenide. The eutectic is evaporated from a solid source and retains its composition on condensation on the semiconductor surface.
- rhythmic sedimentation
- rhythmite
- rhytidome
- RI
- RIA
- ria
- rib
- Ribbentrop, Joachim von (1893–1946)
- ribbon
- ribbon bomb
- ribbon cable
- ribbon development
- ribbon diagram
- ribbon jasper
- ribbon lakes
- ribbon microphone
- ribbons
- ribbon worm
- riboflavin
- ribonuclease
- ribonucleic acid
- ribonucleoprotein
- ribose
- ribosomal RNA
- ribosome