A colourless liquid cyclic alkene, C5H6; r.d. 0.8021; m.p. –97.2°C; b.p. 40.0°C. It is prepared as a by-product during the fractional distillation of crude benzene from coal tar. It undergoes condensation reactions with ketones to give highly coloured compounds (fulvenes) and readily undergoes polymerization at room temperature to give the dimer, dicyclopentadiene. Cyclopentadiene itself is not an aromatic compound because it does not have the required number of pi electrons. However, removal of a hydrogen atom produces the stable cyclopentadienyl ion, C5H5−, which does have aromatic properties. In particular, the ring can coordinate to positive ions in such compounds as ferrocene.
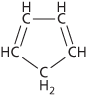
Cyclopentadiene
- FIA
- fiamme
- fiard
- fiat money
- Fibiger, Johannes Andreas Grib
- Fibonacci (1170–1250)
- Fibonacci, Leonardo
- Fibonacci multiplication
- Fibonacci number
- Fibonacci search
- Fibonacci series
- fibratus
- fibre
- Fibre Channel
- Fibre Distributed Data Interface
- fibre distributed data interface
- fibre media
- fibre optic cable
- fibre optic gyroscope
- fibre optics
- fibre-optics system
- fibre to the curb
- fibre to the home
- fibretronics
- fibril