A colourless odourless gas, CO, sparingly soluble in water and soluble in ethanol and benzene; d. 1.25 g dm−3 (0°C); m.p. –199°C; b.p. –191.5°C. It is flammable and highly toxic. In the laboratory it can be made by the dehydration of methanoic acid (formic acid) using concentrated sulphuric acid. Industrially it is produced by the oxidation of natural gas (methane) or (formerly) by the water-gas reaction. It is formed by the incomplete combustion of carbon and is present in car-exhaust gases.
It is a neutral oxide, which burns in air to give carbon dioxide, and is a good reducing agent, used in a number of metallurgical processes. It has the interesting chemical property of forming a range of transition metal carbonyls, e.g. Ni(CO)4. Carbon monoxide is able to use vacant p-orbitals in bonding with metals; the stabilization of low oxidation states, including the zero state, is a consequence of this. This also accounts for its toxicity, which is due to the binding of the CO to the iron in haemoglobin, thereby blocking the uptake of oxygen.
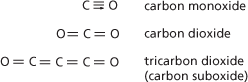
Carbon monoxide
A colourless odourless gas, CO. It is formed by the incomplete combustion of carbon and is present in car-exhaust gases. Carbon monoxide is able to bond with metals; this accounts for its toxicity, which is due to the binding of the CO to the iron in haemoglobin, thereby blocking the uptake of oxygen. In vertebrates small amounts of CO are produced naturally by the enzyme haem oxygenase as a by-product of haem degradation. The gas acts as a neurotransmitter and signalling molecule, and it is also thought to modulate functions of the cardiovascular system and to inhibit the aggregation of platelets. See carboxyhaemoglobin.
- promiscuous mode
- promise
- promontorium
- promoter
- PROM programmer
- prompt
- prompt neutrons
- pronation
- pronghorn
- proof
- proof by contradiction
- proofreading
- proof-theoretic harmony
- proof-theoretic semantics
- proof theory
- proof verification
- propaganda
- propagation
- propagation coefficient(symbol: γ, P)
- propagation delay
- propagation loss
- propagule
- propanal
- propane
- propanedioic acid