A group of phenomena associated with magnetic fields. Whenever an electric current flows a magnetic field is produced; as the orbital motion and the spin of atomic electrons are equivalent to tiny current loops, individual atoms create magnetic fields around them, when their orbital electrons have a net magnetic moment as a result of their angular momentum. The magnetic moment of an atom is the vector sum of the magnetic moments of the orbital motions and the spins of all the electrons in the atom. The macroscopic magnetic properties of a substance arise from the magnetic moments of its component atoms and molecules. Different materials have different characteristics in an applied magnetic field; there are four main types of magnetic behaviour:
1. In diamagnetism the magnetization is in the opposite direction to that of the applied field, i.e. the susceptibility is negative. Although all substances are diamagnetic, it is a weak form of magnetism and may be masked by other, stronger, forms. It results from changes induced in the orbits of electrons in the atoms of a substance by the applied field, the direction of the change (in accordance with Lenz’s law) opposing the applied flux. There is thus a weak negative susceptibility (of the order of −10−8 m3 mol−1) and a relative permeability of slightly less than one.
2. In paramagnetism the atoms or molecules of the substance have net orbital or spin magnetic moments that are capable of being aligned in the direction of the applied field. They therefore have a positive (but small) susceptibility and a relative permeability slightly in excess of one. Paramagnetism occurs in all atoms and molecules with unpaired electrons; e.g. free atoms, free radicals, and compounds of transition metals containing ions with unfilled electron shells. It also occurs in metals as a result of the magnetic moments associated with the spins of the conducting electrons.
3. In ferromagnetic substances, within a certain temperature range, there are net atomic magnetic moments, which line up in such a way that magnetization persists after the removal of the applied field. Below a certain temperature, called the Curie point (or Curie temperature) an increasing magnetic field applied to a ferromagnetic substance will cause increasing magnetization to a high value, called the saturation magnetization. This is because a ferromagnetic substance consists of small (1−0.1 mm across) magnetized regions called domains. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. Within each domain the individual atomic magnetic moments are spontaneously aligned by exchange forces, associated with whether or not the atomic electron spins are parallel or antiparallel. However, in an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. In a very strong field all the domains are lined up in the direction of the field and provide the high observed magnetization. Iron, nickel, cobalt, and their alloys are ferromagnetic. Above the Curie point, ferromagnetic materials become paramagnetic. A variant of exchange is superexchange, i.e. a magnetic interaction that can occur when two magnetic ions are separated by a nonmagnetic ion, with the interaction being mediated by the electrons in the nonmagnetic ion. Superexchange is important in magnetic insulators.
4. Some metals, alloys, and transition-element salts exhibit another form of magnetism called antiferromagnetism. This occurs below a certain temperature, called the Néel temperature, when an ordered array of atomic magnetic moments spontaneously forms in which alternate moments have opposite directions. There is therefore no net resultant magnetic moment in the absence of an applied field. In manganese fluoride, for example, this antiparallel arrangement occurs below a Néel temperature of 72 K. Below this temperature the spontaneous ordering opposes the normal tendency of the magnetic moments to align with the applied field. Above the Néel temperature the substance is paramagnetic.
A special form of antiferromagnetism is ferrimagnetism, a type of magnetism exhibited by the ferrites. In these materials the magnetic moments of adjacent ions are antiparallel and of unequal strength, or the number of magnetic moments in one direction is greater than those in the opposite direction. By suitable choice of rare-earth ions in the ferrite lattices it is possible to design ferrimagnetic substances with specific magnetizations for use in electronic components. See also geomagnetism.
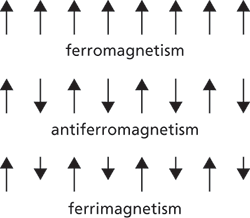
Magnetism. The arrows represent the direction of magnetic moments.
http://www.kayelaby.npl.co.uk/general_physics/2_6/2_6_6.html Values for the magnetic properties of materials at the NPL website
A group of phenomena associated with magnetic fields. Whenever an electric current flows a magnetic field is produced; as the orbital motion and the spin of atomic electrons are equivalent to tiny current loops, individual atoms create magnetic fields around them, when their orbital electrons have a net magnetic moment as a result of their angular momentum. The magnetic moment of an atom is the vector sum of the magnetic moments of the orbital motions and the spins of all the electrons in the atom. The macroscopic magnetic properties of a substance arise from the magnetic moments of its component atoms and molecules. Different materials have different characteristics in an applied magnetic field; there are four main types of magnetic behaviour:
(a) In diamagnetism the magnetization is in the opposite direction to that of the applied field, i.e. the susceptibility is negative. Although all substances are diamagnetic, it is a weak form of magnetism and may be masked by other, stronger, forms. It results from changes induced in the orbits of electrons in the atoms of a substance by the applied field, the direction of the change opposing the applied flux. There is thus a weak negative susceptibility (of the order of –10−8 m3 mol−1) and a relative permeability of slightly less than one.
(b) In paramagnetism the atoms or molecules of the substance have net orbital or spin magnetic moments that are capable of being aligned in the direction of the applied field. They therefore have a positive (but small) susceptibility and a relative permeability slightly in excess of one. Paramagnetism occurs in all atoms and molecules with unpaired electrons; e.g. free atoms, free radicals, and compounds of transition metals containing ions with unfilled electron shells. It also occurs in metals as a result of the magnetic moments associated with the spins of the conducting electrons.
(c) In ferromagnetic substances, within a certain temperature range, there are net atomic magnetic moments, which line up in such a way that magnetization persists after the removal of the applied field. Below a certain temperature, called the Curie point (or Curie temperature) an increasing magnetic field applied to a ferromagnetic substance will cause increasing magnetization to a high value, called the saturation magnetization. This is because a ferromagnetic substance consists of small (1–0.1 mm across) magnetized regions called domains. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. Within each domain the individual atomic magnetic moments are spontaneously aligned by exchange forces, related to whether or not the atomic electron spins are parallel or antiparallel. However, in an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. In a very strong field all the domains are lined up in the direction of the field and provide the high observed magnetization. Iron, nickel, cobalt, and their alloys are ferromagnetic. Above the Curie point, ferromagnetic materials become paramagnetic.A particular type of exchange interaction is superexchange, i.e. a magnetic interaction that can occur when two magnetic ions are separated by a nonmagnetic ion, with the interaction being mediated by the electrons in the nonmagnetic ion. Superexchange is important in magnetic insulators.
(d) Some metals, alloys, and transition-element salts exhibit another form of magnetism called antiferromagnetism. This occurs below a certain temperature, called the Néel temperature, when an ordered array of atomic magnetic moments spontaneously forms in which alternate moments have opposite directions. There is therefore no net resultant magnetic moment in the absence of an applied field. In manganese fluoride, for example, this antiparallel arrangement occurs below a Néel temperature of 72 K. Below this temperature the spontaneous ordering opposes the normal tendency of the magnetic moments to align with the applied field. Above the Néel temperature the substance is paramagnetic.
A special form of antiferromagnetism is ferrimagnetism, a type of magnetism exhibited by the ferrites. In these materials the magnetic moments of adjacent ions are antiparallel and of unequal strength, or the number of magnetic moments in one direction is greater than those in the opposite direction. By suitable choice of rare-earth ions in the ferrite lattices it is possible to design ferrimagnetic substances with specific magnetizations for use in electronic components.
The phenomena associated with regions containing magnetic flux. Magnetic properties were first noticed in the naturally occurring oxide of iron, magnetite. Ampère discovered that a small coil carrying a current behaves like a magnet; he suggested that the origin of all magnetism lay in small circulating currents associated with each atom. Ampère’s theory, which gave a natural explanation of the fact that no isolated magnetic pole had ever been observed, is essentially similar to modern atomic theory: his elementary current circuits (Amperean currents) are the motions of the negatively charged electrons in closed orbits around the positively charged atomic nucleus.
All materials exhibit magnetic properties, the nature of those properties depending on the distribution of electrons in the outer orbits of the atoms. Diamagnetism is a weak effect, common to all materials and resulting from the orbital motion of the atomic electrons. Paramagnetism occurs in certain materials that have a permanent molecular magnetic moment due to electron spin. It is a stronger effect than diamagnetism, opposed to it, and masks it in paramagnetic materials. Some paramagnetic materials, such as iron, also display ferromagnetism (see also ferrimagnetism, antiferromagnetism) at temperatures below the Curie point. Ferromagnetic materials can produce a substantial magnetic flux density and some are suitable for use as permanent magnets. A magnetic field can also be produced by a flowing electric current (see electromagnet).
- Patel, Sardar Vallabhbhai (1875–1950)
- patent
- patera
- paternalism
- paternoster lake
- Paterson’s Curse
- path
- Pathan
- path analysis
- path component
- path-connected
- path dependence
- path-dependent
- pathetic fallacy
- Pathet Lao
- path(in a graph)
- path integral
- path integral formulation
- pathlength
- path loss
- path name
- path name separator
- pathogen
- pathogen-associated molecular pattern
- pathogenesis-related proteins