One of the very large number of possible spatial arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. In the case of ethane, H3C–CH3, one methyl group can rotate relative to the other. There are two extreme cases. In one, the C–H bonds on one group align with the C–H bonds on the other (as viewed along the C–C bond). This is an eclipsed conformation (or eclipsing conformation) and corresponds to a maximum in a graph of potential energy against rotation angle. In the other the C–H bonds on one group bisect the angle between two C–H bonds on the other. This is a staggered conformation (or bisecting conformation) and corresponds to a minimum in the potential-energy curve. In the case of ethane, the energy difference between the two conformations is small (2.8 kcal/mole) and effective free rotation occurs under normal conditions.
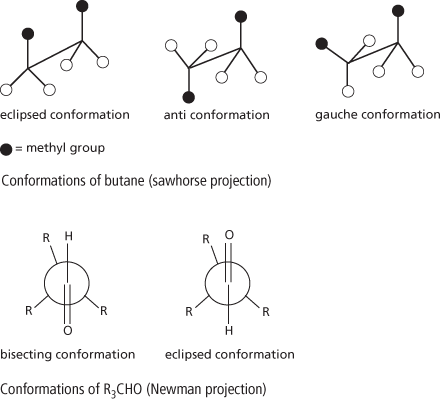
In the case of butane, rotation can occur about the bond between the second and third carbon atoms, MeH2C–CH2Me. The highest maximum occurs when the methyl groups are eclipsed (they are at their closest). Maxima of lower energies occur when the methyl groups on one carbon atom eclipse the hydrogen atoms on the other. The lowest potential energy occurs when a C–Me bond bisects the angle between the C–H bonds (i.e. the two methyl groups are furthest apart). This is called the anti conformation. A lesser minimum occurs when the methyl group bisects the angle between a C–H bond and a C–Me bond. This arrangement is called the gauche conformation.
Similar rotational arrangements occur in other types of molecule. For instance, in a compound such as R3C–CHO, with a C = O double bond, one conformation has the C = O bond bisecting the angle between C–R bonds. This is called the bisecting conformation (note that the C–H bond eclipses one of the C–R bonds in this case). The other has the C = O bond eclipsing a C–R bond. This is the eclipsed or eclipsing conformation. (In this case the C–H bond bisects the angle between the C–R bonds.)
See also torsion angle; ring conformations.
The spatial arrangement of atoms in a molecule responsible for its shape. There are many possible arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. The conformation of proteins determines their function as in the case of enzymes as biocatalysts and proteins that have therapeutic properties such as insulin.
- government bot
- government debt
- government expenditure
- government failure
- government in exile
- Government National Mortgage Association
- government production
- government regulation
- government spending on real goods and services
- government transfer payments
- governor
- GovSat-1
- Gowers, William Timothy (1963– )
- Gowon, Yakubu (1934)
- GP
- GPIB
- GPIO pin
- GPL
- GPM
- GPR
- G protein
- G-protein-coupled receptor
- GPS
- GPU
- Graaff, Robert Jemison van de