In a nonlinear chain of atoms A–B–C–D, the angle between the plane containing atoms ABC and the plane containing BCD. The torsion angle can have any value from 0° to 180°. If the chain is viewed along the line BC, the torsion angle is positive if the bond AB would have to be rotated in a clockwise sense (less than 180°) to eclipse (i.e. align with) the bond CD. If the rotation of AB has to be in an anticlockwise sense, the torsion angle would be negative.
There is a terminology relating to the steric arrangements of atoms based on the size of the torsion angle (see also conformation). The syn arrangement is one in which the size of the torsion angle is ±90°. The anti arrangement is one with a torsion angle between ±90° and 180°. Another distinction is between clinal arrangements (value between 30° and 150° or –30° and –150°) and periplanar arrangements (value between 0 and ±30° or ±150° and 180°). Combining the two gives four ranges of torsion angle in an arrangement:
synperiplanar (sp) 0° to ±30°;
synclinal (sc) 30° to 90° or –30° to –90°;
anticlinal (ac) 90° to 150° or –90° to –150°;
antiperiplanar (ap) ±150° to 180°.
The antiperiplanar conformation is also called a trans conformation; the synperiplanar conformation corresponds to a cis conformation. The synclinal conformation is also called a gauche or skew conformation.
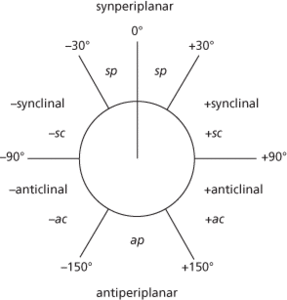
Torsion angle
- umbrisols
- UML
- umland
- unabsorbed field strength
- Unamuno, Miguel de (1864–1936)
- unanimity rule
- unary operation
- UNASUR
- unauthorized colony
- unavailable water
- unbalanced cable
- unbalanced two-port network
- unbiased
- unbiased estimate
- unbiased estimator
- unbiased expectations hypothesis
- unbounded
- unbound moisture
- unbundling
- uncertainty
- uncertainty principle
- uncinus
- unclocked flip-flop
- uncommitted logic array
- uncompetitive