A technique for separating components of an azeotrope by adding a third liquid to form a new azeotrope with one of the original components. It is most commonly used to separate ethanol from water, adding benzene to associate with the ethanol.
A method of separating azeotropic mixtures by distillation in which conventional distillation is often not suitable or possible. It is used for mixtures that have a relative volatility near unity or which form azeotropes and would otherwise require large numbers of theoretical plates and high reflux ratios See superfractionation. It is therefore necessary to increase the relative volatility, which entails an increase in the non-ideality of the mixture. An entrainer is therefore added which is fairly volatile and forms an azeotrope, with one or more of the original components, and leaves overhead, allowing one component to leave in a fairly pure state at the bottom. This new azeotrope must be either heterogeneous or readily separable by some other means such as by liquid–liquid extraction.
The typical layout with heterogeneity (usually on cooling, see Fig. 3) consists of an overhead heterogeneous azeotrope in which there is an entrainer-rich layer that is returned to the column and an A-rich layer that is sent to an entrainer recovery column. The latter produces more azeotrope overhead, which is sent to the common condenser-cooler, and A of the desired purity leaves at the bottom. The A-rich layer is not necessarily the upper layer in the decanter, and an addition of entrainer to the column may have to be made to make up for losses. An example of this system is the use of butyl acetate (entrainer) to remove water (A) from acetic acid (B). Without the entrainer the relative volatility is very low.
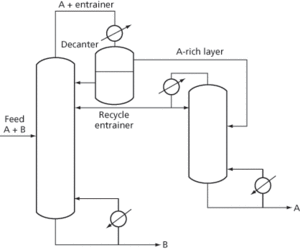
Fig. 3 Azeotropic distillation with heterogeneity
Another example is the use of cyclohexane to separate isopropyl alcohol (IPA) and water in which crude IPA is pumped to the first tower or ‘dryer’ and cyclohexane and water leaves the top of the tower, is condensed, and separates into two layers. Cyclohexane in the top layer is sent as reflux to the tower and the lower water aqueous IPA layer pumped to another tower for part water removal. The amount of cyclohexane in the system is regulated by the level in the reflux drum.
If heterogeneity does not occur, some other means of splitting the A-entrainer azeotrope is required such as liquid–liquid extraction (see Fig. 4).
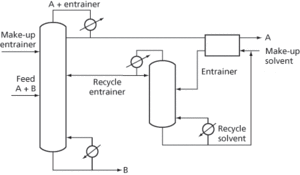
Fig. 4 Azeotropic distillation without heterogeneity
- export concentration
- export control
- export credit
- export credit agency
- Export Credits Guarantee Department
- export incentives
- export-led growth
- export list
- export promotion
- exports
- export subsidy
- export surplus
- Export–Import Bank
- exposed terminal problem
- ex post
- exposure
- exposure age
- exposure meter
- exposure to risk
- express
- ExpressCard
- expressed sequence tag
- expression
- expression of requirements
- expression vector