Organic compounds that contain the group –CHO (the aldehyde group; i.e. a carbonyl group (C = O) with a hydrogen atom bound to the carbon atom). In systematic chemical nomenclature, aldehyde names end with the suffix -al. Examples of aldehydes are methanal (formaldehyde), HCOH, and ethanal (acetaldehyde), CH3CHO. Aldehydes are formed by oxidation of primary alcohols; further oxidation yields carboxylic acids. They are reducing agents and tests for aldehydes include Fehling’s test and Tollens reagent. Aldehydes have certain characteristic addition and condensation reactions. With sodium hydrogensulphate(IV) they form addition compounds of the type [RCOH(SO3)H]- Na+. Formerly these were known as bisulphite addition compounds. They also form addition compounds with hydrogen cyanide to give cyanohydrins and with alcohols to give acetals and undergo condensation reactions to yield oximes, hydrazones, and semicarbazones. Aldehydes readily polymerize. See also ketones.
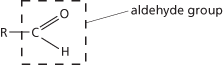
Aldehyde
https://www.acdlabs.com/iupac/nomenclature/93/r93_449.htm Information about IUPAC nomenclature
- Paterson’s Curse
- path
- Pathan
- path analysis
- path component
- path-connected
- path dependence
- path-dependent
- pathetic fallacy
- Pathet Lao
- path(in a graph)
- path integral
- path integral formulation
- pathlength
- path loss
- path name
- path name separator
- pathogen
- pathogen-associated molecular pattern
- pathogenesis-related proteins
- pathogenicity island
- pathology
- path testing
- path vector
- Patiño, Simon Iture (1860–1947)