A type of interaction between molecules occurring in molecules that have hydrogen atoms bound to electronegative atoms (F, N, O). It can be regarded as a strong dipole-dipole attraction caused by the electron-withdrawing properties of the electronegative atom. Thus, in the water molecule the oxygen atom attracts the electrons in the O-H bonds. The hydrogen atom has no inner shells of electrons to shield the nucleus, and there is an electrostatic interaction between the hydrogen proton and a lone pair of electrons on an oxygen atom in a neighbouring molecule. Each oxygen atom has two lone pairs and can make hydrogen bonds to two different hydrogen atoms. The strengths of hydrogen bonds are about one tenth of the strengths of normal covalent bonds. Hydrogen bonding does, however, have significant effects on physical properties. Thus it accounts for the unusual properties of water and for the relatively high boiling points of H2O, HF, and NH3 (compared with H2S, HCl, and PH3). It is also of great importance in living organisms. Hydrogen bonding occurs between bases in the chains of DNA. It also occurs between the C=O and N-H groups in proteins, and is responsible for maintaining the secondary structure. Hydrogen bonds are not purely electrostatic and can be shown to have some covalent character.
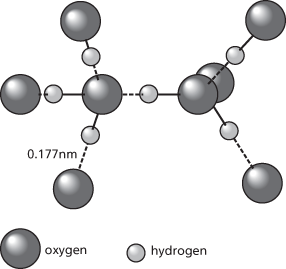
Hydrogen bond
A type of electrostatic interaction between electronegative (fluorine, nitrogen, or oxygen) atoms in one molecule and hydrogen atoms bound to electronegative atoms in another molecule. It is a strong dipole-dipole attraction caused by the electron-withdrawing properties of the electronegative atom. Thus, in the water molecule the oxygen atom attracts the electrons in the O–H bonds. The hydrogen atom has no inner shells of electrons to shield the nucleus, and there is an electrostatic interaction between the hydrogen proton and a lone pair of electrons on an oxygen atom in a neighbouring molecule. Each oxygen atom has two lone pairs and can make hydrogen bonds to two different hydrogen atoms. The strengths of hydrogen bonds are about one tenth of the strengths of normal covalent bonds. Hydrogen bonding does, however, have significant effects on physical properties. Thus it accounts for the unusual properties of water and for its relatively high boiling point. It is also of great importance in living organisms. Hydrogen bonding occurs between complementary base pairs in the two polynucleotide strands of DNA (see base pairing). It also occurs between the C=O and N–H groups in proteins, and is responsible for maintaining the secondary structure.
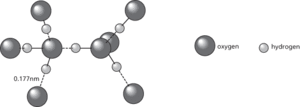
Hydrogen bonds (shown as dotted lines) between water molecules
- Commonwealth
- neural crest cells
- neural net
- neural nets
- neural network
- neural plate
- neural spine
- neural tube
- Neurath, Otto (1882–1945)
- Neurath’s boat
- neuregulin
- neurilemma cell
- neurite
- neurodynamics
- neuroelectricity
- neuroendocrine system
- neurofibril
- neurofilament
- neurogaming
- neuroglia
- neurohaemal organ
- neurohormone
- neurohypophysis
- neuroimaging
- neurolinguistics