A type of reaction in which an alkyl group (from a haloalkane) or an acyl group (from an acyl halide) is substituted on a benzene ring (see illustration). The product is an alkylbenzene (for alkyl substitution) or an alkyl aryl ketone (for acyl substitution). The reactions occur at high temperature (about 100°C) with an aluminium chloride catalyst. The catalyst acts as an electron acceptor for a lone pair on the halide atom. This polarizes the haloalkane or acyl halide, producing a positive charge on the alkyl or acyl group. The mechanism is then electrophilic substitution. Alcohols and alkenes can also undergo Friedel–Crafts reactions. The reaction is named after the French chemist Charles Friedel (1832–99) and the US chemist James M. Craft (1839–1917), who discovered it in 1877.
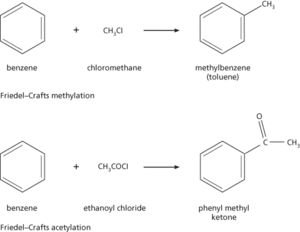
Friedel–Crafts reactions
- pseudoprime
- pseudopunctate
- pseudorandom
- pseudo-random numbers
- pseudorandom numbers
- pseudo-scalar
- pseudosection
- pseudospar
- pseudo-statement
- Pseudosycidium
- pseudotachylite
- pseudovalue
- pseudo-vector
- PSG
- PSI
- psi
- psilocin
- psilocybin
- psilomelane
- Psilonichnus
- Psilophytales
- psilophytes
- Psilopsida
- Psilotum
- psi particle