A cycle of operations in a heat engine. The Rankine cycle approximates more closely to the cycle of a real steam engine than does the Carnot cycle. It therefore predicts a lower ideal thermal efficiency than does the Carnot cycle. In the Rankine cycle, heat is added at constant pressure p1, at which water is converted in a boiler to superheated steam; the steam expands at constant entropy to a pressure p2 in the cylinder; heat is rejected at constant pressure p2 in a condenser; the water so formed is compressed at constant entropy to pressure p1 by a feed pump. The cycle was devised by the Scottish engineer William John Macquorn Rankine (1820–72) in the 1850s.
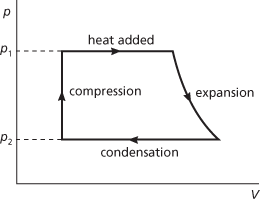
Rankine cycle.
An ideal reversible thermodynamic cycle used in steam power plants (see Fig. 49) that more closely approximates to the cycle of a real steam engine than the Carnot cycle and converts heat into mechanical work. It involves water being introduced under pressure into a boiler and evaporation taking place, followed by expansion of the vapour without the loss of heat, ending in condensation. The cycle therefore consists of four stages: i) steam passes from the boiler to the cylinder at constant pressure; ii) the steam expands adiabatically to the condenser pressure; iii) heat is given to the condenser at constant temperature; iv) condensation is completed and the condensate is returned to the boiler. In the Rankine cycle, the work done is equivalent to the total heat in the steam at the end of the adiabatic expansion subtracted from the total heat in the steam at the beginning of the expansion. The heat supplied is equal to the sensible heat in the condensed steam subtracted from the total heat.
The Rankine cycle is used to describe the way steam-operated heat engines that are found in thermal power generating plants generate power for which the heat sources are nuclear fission, or the combustion of coal, oil, or gas. It is named after Scottish civil engineer and physicist William John Macquorn Rankine (1820–72).
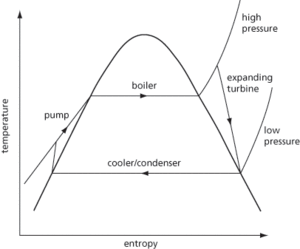
Fig. 49
- maximal ideal
- maximal information coefficient
- maximally flat
- maximal matching
- maximal torus
- Maximilian (1832–67)
- Maximilian I (1459–1519)
- maximin
- maximin principle
- maximin strategy
- maximize
- maximum
- maximum allowable working pressure
- maximum and minimum thermometer
- maximum drying rate
- maximum-entropy method
- maximum flow/minimum cut theorem
- maximum-length sequence
- maximum likelihood
- maximum-likelihood classification
- maximum-likelihood decoding
- maximum likelihood estimate
- maximum likelihood estimator
- maximum likelihood, method of
- maximum likelihood sequence estimation