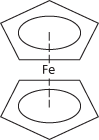
An orange-red crystalline solid, Fe(C5H5)2; m.p. 173°C. It can be made by adding the ionic compound Na+C5H5− (cyclopentadienyl sodium, made from sodium and cyclopentadiene) to iron(III) chloride. In ferrocene, the two rings are parallel, with the iron ion sandwiched between them (hence the name sandwich compound: see formula). The bonding is between pi orbitals on the rings and d-orbitals on the Fe2+ ion. The compound can undergo electrophilic substitution on the C5H5 rings (they have some aromatic character). It can also be oxidized to the blue ion (C5H5)2Fe+. Ferrocene is the first of a class of similar complexes called sandwich compounds. Its systematic name is di-π-cyclopentadienyl iron(II).
Ferrocene
- flash flood
- flash freezing
- flash memory
- flash memory controller
- range(of a function or mapping)
- range of a good or service
- Ranger
- range theory of probability
- range tracking
- range zone
- Ranjit Singh (1780–1839)
- rank
- rank correlation
- rank correlation coefficient
- rank dependent expected utility theory
- ranker
- Rankine cycle
- Rankine scale
- Rankine temperature scale
- ranking
- ranking of towns
- ranking service
- rank(in statistics)
- rankit
- rank-nullity theorem