A distillation technique in which a solvent is added to the mixture in order to separate two closely boiling components. The added solvent is usually nonvolatile and is selected for its ability to have different effects on the volatilities of the components.
The separation process used for azeotropic mixtures in which a solvent is added, leaving the bottom of the column carrying one component with it, allowing the other component to leave at the top of the column (see Fig. 21). Extractive distillation is more widely used than azeotropic distillation since there is a wider choice of agent and there is no need for close matching of volatilities. Azeotropic entrainers must usually boil within 5–20°C of the other components. There is also a lower heat requirement since the solvent is not vaporized. There is also a wider choice of operating conditions. The solvent/feed ratio, for example, is not critical, unlike an entrainer/feed ratio, and there is easier recovery of the agent. Solvents are cheap, readily obtainable, non-corrosive, non-toxic, unreactive, thermally stable, and easily recoverable, as well as being readily miscible, and have a low volatility. The solvent is added a few trays down from the top of the column with the trays above the solvent feed point functioning as a solvent recovery section to remove traces of solvent from the overhead vapour A. The solvent leaves the bottom of the main column together with component B, from which it is usually readily separated in a solvent-recovery column in view of its presumed low volatility. The solvent is recycled to the main column with a make-up stream being provided to allow for any solvent losses. Examples include the separation of paraffins (A) from toluene (B) using phenol, and isobutane (A) from butane (B) using furfural.
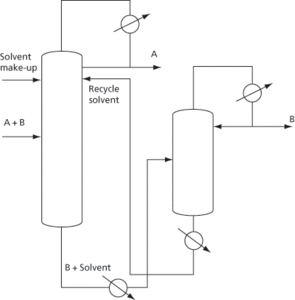
Fig. 21
- return vehicle
- retweet
- reurbanization
- reusable launch vehicle
- reusable resource
- reusable software
- Reuter, Paul Julius, Baron von (1816–99)
- Reuven Ramaty High-Energy Solar Spectroscopic Imager
- revaluation
- revamp
- revealed preference
- revealed preference pricing
- revealed theology
- revegetation
- revelation
- Revelle, Roger
- revenge
- revenue
- revenue tariff
- reverberation
- reverberation time
- reverberatory furnace
- Revere, Paul (1735–1818)
- reversal
- reversal function