Compounds with large three-dimensional molecular structures containing ether chains linked by three-coordinate nitrogen atoms. Thus cryptands are macropolycyclic polyaza-polyethers. For example, the compound (2,2,2)-cryptand has three chains of the form
These chains are linked at each end by a nitrogen atom. Cryptands, like the crown ethers, can form coordination complexes with ions that can fit into the cavity formed by the open three-dimensional structure, i.e. they can ‘cryptate’ the ion. Various types of cryptand have been produced having both spherical and cylindrical cavities. The cryptands have the same kind of properties as the crown ethers and the same uses. In general, they form much more strongly bound complexes and can be used to stabilize unusual ionic species. For example, it is possible to produce the negative Na− ion in the compound [(2,2,2)-cryptand-Na]+Na−, which is a gold-coloured crystalline substance stable at room temperature. Cluster ions, such as Pb52−, can be similarly stabilized.
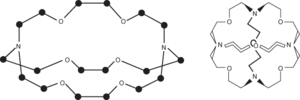
Cryptands
- UT
- UTC
- uterine cycle
- uterus
- UTF-16
- UTF-32
- UTF-8
- Uthman (574–656)
- Uthman dan Fodio (1754–1817)
- util
- utilitarianism
- utilities
- utility
- utility calculus
- utility computing
- utility flow diagram
- utility function
- utility maximization
- utility possibility frontier
- utility programs
- utility waste
- utopia
- Utopia Planitia
- UTP
- Utrecht, Peace of (1713)