A region in which an electron may be found in an atom or molecule. In the original Bohr theory of the atom the electrons were assumed to move around the nucleus in circular orbits (and in elliptical orbits in generalizations of the Bohr theory), but further advances in quantum mechanics led to the view that it is not possible to give a definite path for an electron. According to wave mechanics, the electron has a certain probability of being in a given element of space. Thus, for a hydrogen atom, the electron can be anywhere from close to the nucleus to out in space, but the maximum probability in spherical shells of equal thickness occurs in a spherical shell around the nucleus with a radius equal to the radius of the atom predicted by the Bohr theory. The probabilities of finding an electron in different regions can be obtained by solving the Schrödinger wave equation to give the wave function ψ, and the probability of location per unit volume is then proportional to |ψ|2. Thus the idea of electrons in fixed orbits has been replaced by that of a probability distribution around the nucleus—an atomic orbital. Alternatively, the orbital can be thought of as an electric charge distribution (averaged over time). In representing orbitals, it is convenient to take a surface enclosing the space in which the electron is likely to be found with a high probability.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l=0). This is spherical. There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l. For instance, p-orbitals each have two lobes; most d-orbitals have four lobes.
In molecules, the valence electrons move under the influence of two nuclei (in a bond involving two atoms) and there are corresponding molecular orbitals for electrons. It is convenient in considering these to regard them as formed by overlap of atomic orbitals. In a hydrogen molecule, the s-orbitals on the two atoms overlap and form a molecular orbital between the two nuclei. This is an example of a sigma orbital. In a double bond, as in ethene, one bond is produced by overlap along the line of axes to form a sigma orbital. The other is produced by sideways overlap of the lobes of the p-orbitals. The resulting molecular orbital has two parts, one on each side of the sigma orbital—this is a pi orbital. It is also possible for a delta orbital to form by lateral overlap of two d-orbitals. In fact, the combination of two atomic orbitals produces two molecular orbitals with different energies. The one of lower energy is the bonding orbital, holding the atoms together; the other is the antibonding orbital, which would tend to push the atoms apart. In the case of valence electrons, only the lower (bonding) orbital is filled.
In considering the formation of molecular orbitals it is often useful to think in terms of hybrid atomic orbitals. For instance, carbon has in its outer shell one s-orbital and three p-orbitals. In forming methane (or other tetrahedral molecules) these can be regarded as combining to give four equivalent sp3 hybrid orbitals, each with a lobe directed to a corner of a tetrahedron. It is these that overlap with the s-orbitals on the hydrogen atoms. In ethene, two p-orbitals combine with the s-orbital to give three sp2 hybrids with lobes in a plane pointing to the corners of an equilateral triangle. These form the sigma orbitals in the C–H and C–C bonds. The remaining p-orbitals (one on each carbon) form the pi orbital. In ethyne, sp2 hybridization occurs to give two hybrid orbitals on each atom with lobes pointing along the axis. The two remaining p-orbitals on each carbon form two pi orbitals. Hybrid atomic orbitals can also involve d-orbitals. For instance, square-planar complexes use sp2d hybrids; octahedral complexes use sp3d2.
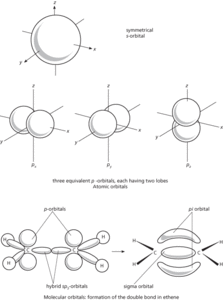
Orbitals.
A region in which an electron may be found in an atom or molecule. In the original Bohr theory of the atom the electrons were assumed to move around the nucleus in circular orbits, but further advances in quantum mechanics led to the view that it is not possible to give a definite path for an electron. According to wave mechanics, the electron has a certain probability of being in a given element of space. Thus for a hydrogen atom the electron can be anywhere from close to the nucleus to out in space but the maximum probability in spherical shells of equal thickness occurs in a spherical shell around the nucleus with a radius equal to the Bohr radius of the atom. The probabilities of finding an electron in different regions can be obtained by solving the Schrödinger wave equation to give the wave function ψ, and the probability of location per unit volume is then proportional to |ψ| 2. Thus the idea of electrons in fixed orbits has been replaced by that of a probability distribution around the nucleus – an atomic orbital. Alternatively, the orbital can be thought of as an electric charge distribution (averaged over time). In representing orbitals it is convenient to take a surface enclosing the space in which the electron is likely to be found with a high probability.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). This is spherical. There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l. For instance, p-orbitals each have two lobes; most d-orbitals have four lobes.
In molecules, the valence electrons move under the influence of two nuclei (in a bond involving two atoms) and there are corresponding molecular orbitals for electrons. It is convenient in considering these to regard them as formed by overlap of atomic orbitals. In a hydrogen molecule the s-orbitals on the two atoms overlap and form a molecular orbital between the two nuclei. This is an example of a sigma orbital. In a double bond, as in ethene, one bond is produced by overlap along the line of axes to form a sigma orbital. The other is produced by sideways overlap of the lobes of the p-orbitals. The resulting molecular orbital has two parts, one on each side of the sigma orbital – this is a pi orbital. It is also possible for a delta orbital to form by lateral overlap of two d-orbitals. In fact, the combination of two atomic orbitals produces two molecular orbitals with different energies. The one of lower energy is the bonding orbital, holding the atoms together; the other is the antibonding orbital, which would tend to push the atoms apart. In the case of valence electrons, only the lower (bonding) orbital is filled.
In considering the formation of molecular orbitals it is often useful to think in terms of hybrid atomic orbitals. For instance, carbon has in its outer shell one s-orbital and three p-orbitals. In forming methane (or other tetrahedral molecules) these can be regarded as combining to give four equivalent sp3 hybrid orbitals, each with a lobe directed to a corner of a tetrahedron. It is these that overlap with the s-orbitals on the hydrogen atoms. In ethene, two p-orbitals combine with the s-orbital to give three sp2 hybrids with lobes in a plane pointing to the corners of an equilateral triangle. These form the sigma orbitals in the C-H and C-C bonds. The remaining p-orbitals (one on each carbon) form the pi orbital. In ethyne, sp hybridization occurs to give two hybrid orbitals on each atom with lobes pointing along the axis. The two remaining p-orbitals on each carbon form two pi orbitals. Hybrid atomic orbitals can also involve d-orbitals. For instance, square-planar complexes use sp2d hybrids; octahedral complexes use sp3d2. Orbital hybridization can be discussed using group theory.
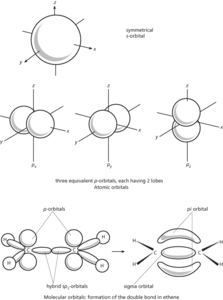
Orbital
- continuous spectrum
- continuous stationery
- continuous stirred-tank reactor
- continuous time
- continuous-time Markov chain
- continuous time process
- continuous-tone image
- continuous variable
- continuous variation
- continuous velocity logging
- continuous wave
- continuous-wave radar
- continuum
- continuum hypothesis
- CONTOUR
- contour
- contour current
- contour diagram
- contour feathers
- contour integration
- contourites
- contour line
- contraception
- contract
- contractarianism