The ability of certain substances to rotate the plane of plane-polarized light as it passes through a crystal, liquid, or solution. It occurs when the molecules of the substance are asymmetric, so that they can exist in two different structural forms each being a mirror image of the other. The two forms are optical isomers or enantiomers. The existence of such forms is also known as enantiomorphism (the mirror images being enantiomorphs). One form will rotate the light in one direction and the other will rotate it by an equal amount in the other. The two possible forms are described as dextrorotatory or laevorotatory according to the direction of rotation, and prefixes d- and l-, respectively, are used to designate the isomer, as in d-tartaric and l-tartaric acids (see formulae). An equimolar mixture of the two forms is not optically active. It is called a racemic mixture (or racemate) and designated by dl-. Prefixes are used to designate the isomer: (+)- (dextrorotatory), (−)- (laevorotatory), and (±)- (racemic mixture) are now preferred to, and increasingly used for, the former d-, l-, and dl-, respectively. In addition, certain molecules can have a meso form in which one part of the molecule is a mirror image of the other. Such molecules are not optically active.
Molecules that show optical activity have no plane of symmetry. The commonest case of this is in organic compounds in which a carbon atom is linked to four different groups. An atom of this type is said to be a chiral centre. Asymmetric molecules showing optical activity can also occur in inorganic compounds. For example, an octahedral complex in which the central ion coordinates to six different ligands would be optically active. Many naturally occurring compounds show optical isomerism and usually only one isomer occurs naturally. For instance, glucose is found in the dextrorotatory form. The other isomer, l- or (−)-glucose, can be synthesized in the laboratory, but cannot be synthesized by living organisms.
Optical activity is understood in terms of the quantum theory of the interaction between light and electrons in molecules.
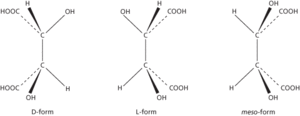
Optical activity. Isomers of tartaric acid.
The ability of certain substances to rotate the plane of plane-polarized light as it passes through a crystal, liquid, or solution. It occurs when the molecules of the substance are asymmetric, so that they can exist in two different structural forms each being a mirror image of the other (see chirality element). The two forms are optical isomers or enantiomers. The existence of such forms is also known as enantiomorphism (the mirror images being enantiomorphs). One form will rotate the light in one direction and the other will rotate it by an equal amount in the other. The two possible forms are described as dextrorotatory or laevorotatory according to the direction of rotation. Prefixes are used to designate the isomer: (+)- (dextrorotatory), (–)- (laevorotatory), and (±)- (racemic mixture) are now preferred to, and increasingly used for, the former d-, l-, and dl-, respectively. An equimolar mixture of the two forms is not optically active. It is called a racemic mixture (or racemate). In addition, certain molecules can have a meso form in which one part of the molecule is a mirror image of the other. Such molecules are not optically active.
Molecules that show optical activity have no plane of symmetry. The commonest case of this is in organic compounds in which a carbon atom is linked to four different groups. An atom of this type is said to be a chiral centre. Asymmetric molecules showing optical activity can also occur in inorganic compounds. For example, an octahedral complex in which the central ion coordinates to six different ligands would be optically active. Many naturally occurring compounds show optical isomerism and usually only one isomer occurs naturally. For instance, glucose is found in the dextrorotatory form. The other isomer, (–)- or l-glucose, can be synthesized in the laboratory, but cannot be synthesized by living organisms. Optical activity can be described in terms of the interaction between molecules and electromagnetic fields. See also absolute configuration.
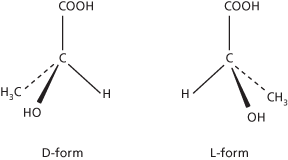
Optical activity
The ability of certain substances to rotate the plane of plane-polarized light as it passes through a crystal, liquid, or solution. It occurs when the molecules of the substance are asymmetric, so that they can exist in two different structural forms each being a mirror image of the other. The two forms are optical isomers or enantiomers (see illustration). The existence of such forms is also known as enantiomorphism (the mirror images being enantiomorphs). One form will rotate the light in one direction and the other will rotate it by an equal amount in the other. The two possible forms are described as dextrorotatory or laevorotatory according to the direction of rotation, and the prefixes (+)- and (−)- are used, respectively, to designate the isomer, as in (+)-tartaric and (−)-tartaric acids. (The prefixes d- and l- are now obsolete.) An equimolar mixture of the two forms is not optically active. It is called a racemic mixture (or racemate) and designated by (+)-. In addition, certain molecules can have a meso form in which one part of the molecule is a mirror image of the other. Such molecules are not optically active.
Molecules that show optical activity have no plane of symmetry. The commonest case of this is in organic compounds in which a carbon atom is linked to four different groups. An atom of this type is said to be a chiral centre. Many naturally occurring compounds show optical isomerism and usually only one isomer occurs naturally. For instance, glucose is found in the dextrorotatory form. The other isomer, (−)-glucose, can be synthesized in the laboratory, but cannot be synthesized by living organisms.
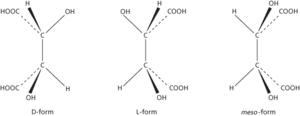
Optical activity: isomers of tartaric acid