The temperature and pressure at which the vapour, liquid, and solid phases of a substance are in equilibrium. For water the triple point occurs at 273.16 K and 611.2 Pa. This value forms the basis of the definition of the kelvin and the thermodynamic temperature scale.
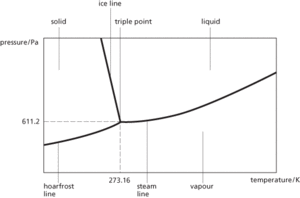
Triple point. The triple point of water.
The temperature and pressure at which the vapour, liquid, and solid phases of a substance are in equilibrium. For water the triple point occurs at 273.16 K and 611.2 Pa. This value forms the basis of the definition of the kelvin and the thermodynamic temperature scale.
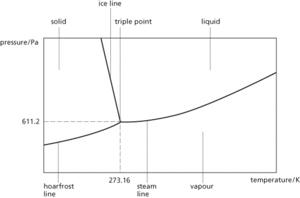
Triple point
The temperature and pressure at which the gas, liquid, and solid phases (or states) of a substance are in equilibrium. The triple point of water, in which vapour, liquid, and ice phases exist in equilibrium, is 0.01ºC and 611.2 Pa. The triple point of water forms the basis of the thermodynamic temperature scale proposed by Lord Kelvin.
The point on a phase diagram at which planes or phases meet. This is the temperature and pressure at which the three phases (solid, liquid, and gas) of a substance can coexist (see also kelvin scale). In a solid-solution system, the triple point is usually defined by the original pressure and temperature conditions at which the solid, liquid, and gaseous phases of a substance are in equilibrium.
- Sabatier process
- Sabatier–Senderens process
- Sabik
- Sabines
- Sabine, Sir Edward
- sabkha
- sable
- sabre-toothed tiger
- SAC
- saccade
- saccate
- saccharide
- saccharin
- Saccharomyces
- saccharose
- Saccominopsis
- Sacco–Vanzetti Case
- sacculus
- saccus
- Sacheverell, Henry (1674–1724)
- Sachse process
- Sachse reaction
- Sachs, Julius von (1832–97)
- Sachs–Wolfe effect
- sacking