A graphical presentation of the interaction of three components in a mixture known as a ternary liquid system. The diagram (see Fig. 56) uses equilateral triangular coordinates, with each liquid being presented as either a mass fraction or percentage in terms of the other two. Any point on the side of the diagram represents a binary mixture. Where one pair of liquids is partially soluble in each other and both are fully soluble in the third at a particular temperature, the diagram is known as an isotherm. An example is benzene, water, and acetic acid with benzene dissolving completely in the other two. For example, in Fig. 56a, any ternary liquid outside the solubility curve at P is a homogenous solution of one liquid. Any ternary liquid below at Q will form two insoluble, saturated liquid phases of equilibrium compositions R and S. The line RS is the tie line, which passes through Q. The plait point T is the last tie line and the point where A- and B-rich solubility curves merge. Where the two pairs of liquids are partially soluble, such as chlorobenzene (A) and water (B), and water and methyl ethyl ketone (C), the isotherm appears as Fig. 56b. Homogeneous ternary solutions are formed at P while two liquids phases at equilibrium appear with R and S corresponding to tie lines in the heterogeneous area.
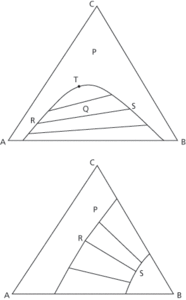
Fig. 56a and 56b
- Hotspur
- hot surface ignition temperature
- hot swapping
- hot tapping
- Hottel charts
- hot towers
- hot-wire ammeter
- hot wire anemometer
- hot-wire gauge
- hot-wire instrument
- hot work
- hot working
- Hounsfield, Sir Godfrey Newbold
- Houphouët-Boigny, Félix (1905–93)
- hour angle
- hour circle
- Hourglass Nebula
- hours of work
- sign digit
- signed
- signed area
- signed field
- signed formula
- signed-magnitude representation
- signed minor