A representation of the structure of a molecule in the form of a diagram showing the bonds between atoms; dots are used to represent valence electrons, with a shared pair of dots between atoms representing a covalent bond. These diagrams were devised by Gilbert Newton Lewis in 1916. Although this representation of chemical bonds was replaced by quantum chemistry, Lewis structures have remained useful, albeit in a limited way.
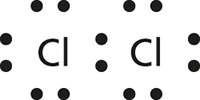
Lewis Structure for the chlorine molecule Cl2
- communicating states
- communication
- communication channel
- communication geography
- communication interface
- Communication/Navigation Outage Forecast System
- communication network
- Communication, Ocean and Meteorological Satellite
- Communication Ocean Meteorological Satellite
- communication port
- communication processor
- communication satellite
- communications carrier assembly
- communication server
- communications satellite
- Communications Satellite Corporation
- communications software
- communication subnet
- communication subnetwork
- communication system
- communication theory
- communism
- Communist Manifesto
- communitarianism
- community