A whitish solid, AlCl3, which fumes in moist air and reacts violently with water (to give hydrogen chloride). It is known as the anhydrous salt (hexagonal; r.d. 2.44 (fused solid); m.p. 190°C (2.5 atm.); sublimes at 178°C) or the hexahydrate AlCl3.6H2O (rhombic; r.d. 2.398; loses water at 100°C), both of which are deliquescent. Aluminium chloride may be prepared by passing hydrogen chloride or chlorine over hot aluminium or (industrially) by passing chlorine over heated aluminium oxide and carbon. The chloride ion is polarized by the small positive aluminium ion and the bonding in the solid is intermediate between covalent and ionic. In the liquid and vapour phases dimer molecules exist, Al2Cl6, in which there are chlorine bridges making coordinate bonds to aluminium atoms (see formula). The AlCl3 molecule can also form compounds with other molecules that donate pairs of electrons (e.g. amines or hydrogen sulphide); i.e. it acts as a Lewis acid. At high temperatures the Al2Cl6 molecules in the vapour dissociate to (planar) AlCl3 molecules. Aluminium chloride is used commercially as a catalyst in the cracking of oils. It is also a catalyst in certain other organic reactions, especially the Friedel–Crafts reaction.
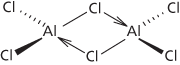
Aluminium chloride
- hard upper torso
- hardware
- hardware address
- hardware character generation
- hardware circuitry
- hardware compilation
- hardware description
- hardware description language
- hardware maintenance
- hardware reliability
- hardware replication
- hardware security
- hardware/software
- hardwired
- hardwired advert
- hardwood
- Hard X-ray Modulation Telescope
- hard X-rays
- Hardy, Godfrey Harold
- Hardy, Godfrey Harold (1877–1947)
- Hardy, Thomas (1752–1832)
- Hardy-Weinberg equilibrium
- Hardy–Weinberg law
- Hardy–Weinberg ratio
- hare