The cycling of sulphur between the biotic (living) and abiotic (nonliving) components of the environment. Most of the sulphur in the abiotic environment is found in rocks, although a small amount is present in the atmosphere as sulphur dioxide (SO2), produced by combustion of fossil fuels. Sulphate (SO42−), derived from the weathering and oxidation of rocks, is taken up by plants and incorporated into sulphur-containing proteins. In this form sulphur is passed along food chains to animals. Decomposition of dead organic matter and faeces by anaerobic sulphate-reducing bacteria returns sulphur to the abiotic environment in the form of hydrogen sulphide (H2S). Hydrogen sulphide can be converted back to sulphate or to elemental sulphur by the action of different groups of photosynthetic and sulphide-oxidizing bacteria. Elemental sulphur becomes incorporated into rocks.
The cycling of sulphur between the biotic (living) and abiotic (nonliving) components of the environment (see biogeochemical cycle). Most of the sulphur in the abiotic environment is found in rocks, although a small amount is present in the atmosphere as sulphur dioxide (SO2), produced by combustion of fossil fuels, and volatile organic sulphur compounds such as dimethyl sulphide, produced by phytoplankton in the oceans. Sulphate (SO42−), derived from the weathering and oxidation of rocks and also found in oceanic sediment, is taken up by plants and phytoplankton and incorporated into sulphur-containing proteins. In this form sulphur is passed along food chains to animals. Decomposition of dead organic matter and faeces by anaerobic sulphate-reducing bacteria returns sulphur to the abiotic environment in the form of hydrogen sulphide (H2S). Hydrogen sulphide can be converted back to sulphate or to elemental sulphur by the action of different groups of photosynthetic and sulphide-oxidizing bacteria (see sulphur bacteria). Elemental sulphur becomes incorporated into rocks.
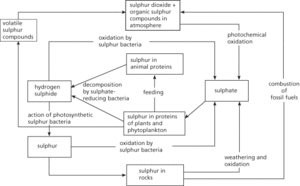
The sulphur cycle
- diffusion equation
- diffusion flame
- diffusion gradient
- diffusion limited aggregation
- diffusion of innovations
- diffusion potential
- diffusion process
- diffusion pump
- diffusive flux
- diffusivity
- différance
- Digby, Kenelm (1603–65)
- digenite
- digest
- digester
- digestion
- digestive system
- Digger
- digit
- digital
- digital ammeter
- digital audio broadcasting
- digital audio tape
- digital camera
- digital cash