A compound in which molecules or ions form coordinate bonds to a metal atom or ion. Often complexes occur as complex ions, such as [Cu(H2O)6]2+ or Fe[(CN)6]3−. A complex may also be a neutral molecule (e.g. PtCl2(NH3)2). The formation of such coordination complexes is typical behaviour of transition metals. The complexes formed are often coloured and have unpaired electrons (i.e. are paramagnetic). It is possible to have polynuclear transition metal complexes. See also ligand; chelate.
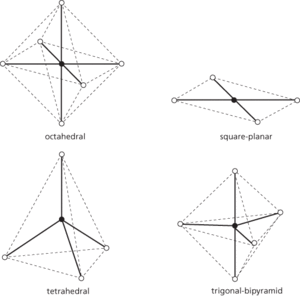
Complexes
See lithostratigraphic unit.
- autological
- autolysis
- automated disk library
- automated econometrics
- automated reasoning
- automated tape library
- automated tensor low-energy electron diffraction
- automated test equipment
- Automated Transfer Vehicle
- automatic brightness control
- automatic coding
- Automatic Computing Engine
- automatic control
- automatic data capture
- automatic data conversion
- linear equation
- linear expansivity
- linear filter
- linear first‐order differential equation
- linear function
- linear grammar
- linear group
- linear independence
- linear interpolation
- linear inverter