1. A distribution of entities or properties arrayed in order of increasing or decreasing magnitude. For example, a beam of ions passed through a mass spectrograph, in which they are deflected according to their charge-to-mass ratios, will have a range of masses called a mass spectrum. A sound spectrum is the distribution of energy over a range of frequencies of a particular source.
2. A range of electromagnetic energies arrayed in order of increasing or decreasing wavelength or frequency (see electromagnetic spectrum). The emission spectrum of a body or substance is the characteristic range of radiations it emits when it is heated, bombarded by electrons or ions, or absorbs photons. The absorption spectrum of a substance is produced by examining, through the substance and through a spectroscope, a continuous spectrum of radiation. The energies removed from the continuous spectrum by the absorbing medium show up as black lines or bands. With a substance capable of emitting a spectrum, these are in exactly the same positions in the spectrum as some of the lines and bands in the emission spectrum.
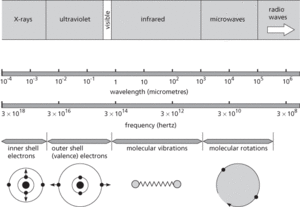
Spectrum. Sources of electromagnetic spectra.
Emission and absorption spectra may show a continuous spectrum, a line spectrum, or a band spectrum. A continuous spectrum contains an unbroken sequence of frequencies over a relatively wide range; it is produced by incandescent solids, liquids, and compressed gases. Line spectra are discontinuous lines produced by excited atoms and ions as they fall back to a lower energy level. Band spectra (closely grouped bands of lines) are characteristic of molecular gases or chemical compounds. See also spectroscopy.
The spectrum of a square matrix A is the sets of its eigenvalues λ or equally those λ for which A–λI is not invertible. More generally, for a continuous (see continuous function) linear map T:V→V on a Banach space V, the spectrum, denoted σ(T), is the set of values λ ε ℂ such that is T–λI has no continuous inverse. This will include any eigenvalues but may also include other values.
1. A range of electromagnetic energies arranged in order of wavelength or frequency (see electromagnetic spectrum). The emission spectrum of a body or substance is the range of radiations it emits when it is heated, is bombarded by electrons or ions, or absorbs photons. The absorption spectrum of a substance consists of dark lines or bands in a continuous spectrum, each line being a wavelength or group of wavelengths at which light is removed from the continuous spectrum by the absorbing medium. These lines and bands are at the same wavelengths as some of the lines and bands in the substance’s emission spectrum. Emission and absorption spectra may show a continuous spectrum (also called a continuum), a line spectrum, or a band spectrum. A continuous spectrum contains an unbroken sequence of frequencies over a wide range; continuous spectra are produced by incandescent solids, liquids, and compressed gases. Line spectra are discontinuous lines produced by excited atoms and ions as they fall back to a lower energy level. Band spectra (closely grouped bands of lines) are characteristic of molecular gases or chemical compounds.
2. The coloured band produced when visible light is passed through a spectroscope. See also spectroscopy.
The pattern of frequencies or wavelengths obtained when electromagnetic radiations are separated into their constituent parts. Visible light is part of the electromagnetic spectrum and most sources emit waves over a range of wavelengths that can be broken up or ‘dispersed’; white light can be separated (for example, using a triangular prism) into red, orange, yellow, green, blue, indigo, and violet. The visible spectrum was first studied by English physicist Isaac Newton, who in 1666 showed how white light could be broken up into different colours.
There are many types of spectra, both emission and absorption, for radiation and particles, used in spectroscopy. An incandescent body gives rise to a continuous spectrum where the dispersed radiation is distributed uninterruptedly over a range of wavelengths. A gaseous element gives a line spectrum—one or more bright discrete lines at characteristic wavelengths. Molecular gases give band spectra in which there are groups of closely-packed lines. In an absorption spectrum dark lines or spaces replace the characteristic bright lines of the absorbing medium. The mass spectrum of an element is obtained from a mass spectrometer and shows the relative proportions of its constituent isotopes.
1. A distribution of entities or properties arrayed in order of increasing or decreasing magnitude. For example, a beam of ions passed through a mass spectrograph, in which they are deflected according to their charge-to-mass ratios, will have a range of masses called a mass spectrum. A sound spectrum is the distribution of energy over a range of frequencies of a particular source.
2. A range of electromagnetic energies arrayed in order of increasing or decreasing wavelength or frequency (see electromagnetic spectrum). The emission spectrum of a body or substance is the characteristic range of radiations it emits when it is heated, bombarded by electron or ions, or absorbs photons. The absorption spectrum of a substance is produced by examining, through the substance and through a spectroscope, a continuous spectrum of radiation. The energies removed from the continuous spectrum by the absorbing medium show up as black lines or bands. With a substance capable of emitting a spectrum, these are in exactly the same positions in the spectrum as some of the lines and bands in the emission spectrum.
Emission and absorption spectra may show a continuous spectrum, a line spectrum, or a band spectrum. A continuous spectrum contains an unbroken sequence of frequencies over a relatively wide range; it is produced by incandescent solids, liquids, and compressed gases. Line spectra are discontinuous lines produced by excited atoms and ions as they fall back to a lower energy level. Band spectra (closely grouped bands of lines) are characteristic of molecular gases or chemical compounds. See also spectroscopy.
The range of possible frequencies that a particular (electrical) signal can have. For example, the audio spectrum is generally considered to extend from 20 hertz to 20 kilohertz, so a given audio signal will be found in this range, and a given instrument will have its own spectrum of frequencies or spectral response within this range.
A range of electromagnetic energies arrayed in order of increasing or decreasing wavelength or frequency. The emission spectrum of a body or substance is the characteristic range of radiations it emits when it is heated, bombarded by electrons or ions, or absorbs photons. The absorption spectrum of a substance is produced by examining, through the substance and through a spectroscope, a continuous spectrum of radiation. The energies removed from the continuous spectrum by the absorbing medium show up as black lines or bands; with a substance capable of emitting a spectrum these are in exactly the same positions in the spectrum as the emission lines and bands would occur in the emission spectrum.
Emission and absorption spectra may show a continuous spectrum, a line spectrum, or a band spectrum. A continuous spectrum contains an unbroken sequence of frequencies over a relatively wide range; it is produced by incandescent solids, liquids, and compressed gases. Line spectra are discontinuous lines produced by excited atoms and ions as they fall back to a lower energy level. Band spectra (closely grouped bands of lines) are characteristic of molecular gases or chemical compounds. Absorption spectra of chlorophylls and other photosynthetic pigments are important in the study of photosynthesis. See action spectrum.
A series of lines (line spectra), produced as electrons return to their original energy levels and emit excess energy as infrared, visible, or ultraviolet light of characteristic wavelengths, after atoms have been heated strongly and valence electrons in the outer shell have moved to higher energy levels. Each element has a characteristic line spectrum. The intensity of each line is related to the concentration of the element being excited.
- Columbanus, St (543–615)
- Columba, St (521–97)
- Columbia
- columbite
- columbium
- Columbus
- Columbus, Christopher (1451–1506)
- Columbus Control Centre
- columella
- column
- columnal
- columnar
- columnar epithelium
- columnar joint
- columnar section
- column chromatography
- column density
- column equivalence
- column-major order
- column operation
- column-ragged
- column rank
- column space
- column vector
- colure