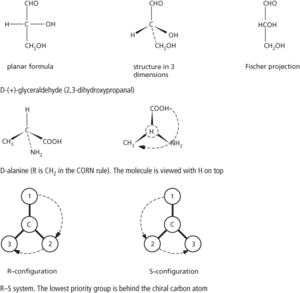
A way of denoting the absolute structure of an optical isomer (see optical activity). Two conventions are in use: The d–l convention relates the structure of the molecule to some reference molecule. In the case of sugars and similar compounds, the dextrorotatory form of glyceraldehyde (HOCH2CH(OH)CHO), 2,3-dihydroxypropanal) was used. The rule is as follows. Write the structure of this molecule down with the asymmetric carbon in the centre, the –CHO group at the top, the –OH on the right, the –CH2OH at the bottom, and the –H on the left. Now imagine that the central carbon atom is at the centre of a tetrahedron with the four groups at the corners and that the –H and –OH come out of the paper and the –CHO and –CH2OH groups go into the paper. The resulting three-dimensional structure was taken to be that of d-glyceraldehyde and called d-glyceraldehyde. Any compound that contains an asymmetric carbon atom having this configuration belongs to the d-series. One having the opposite configuration belongs to the l-series. It is important to note that the prefixes d- and l- do not stand for dextrorotatory and laevorotatory (i.e. they are not the same as d- and l-). In fact the arbitrary configuration assigned to d-glyceraldehyde is now known to be the correct one for the dextrorotatory form, although this was not known at the time. However, all d-compounds are not dextrorotatory. For instance, the acid obtained by oxidizing the –CHO group of glyceraldehyde is glyceric acid (1,2-dihydroxypropanoic acid). By convention, this belongs to the d-series, but it is in fact laevorotatory; i.e. its name can be written as d-glyceric acid or l-glyceric acid. To avoid confusion it is better to use + (for dextrorotatory) and – (for laevorotatory), as in d-(+)-glyceraldehyde and d-(–)-glyceric acid.
The d-l convention can also be used with alpha amino acids (compounds with the –NH2 group on the same carbon as the –COOH group). In this case the molecule is imagined as being viewed along the H-C bond between the hydrogen and the asymmetric carbon atom. If the clockwise order of the other three groups is –COOH, –R, –NH2, the amino acid belongs to the d-series; otherwise it belongs to the l-series. This is known as the CORN rule.
The r–s convention is a convention based on priority of groups attached to the chiral carbon atom. The order of priority is I, Br, Cl, SO3H, OCOCH3, OCH3, OH, NO2, NH2, COOCH3, CONH2, COCH3, CHO, CH2OH, C6H5, C2H5, CH3, H, with hydrogen lowest. The molecule is viewed with the group of lowest priority behind the chiral atom. If the clockwise arrangement of the other three groups is in descending priority, the compound belongs to the r-series; if the descending order is anticlockwise it is in the s-series. d-(+)-glyceraldehyde is r-(+)-glyceraldehyde. See illustration.
Absolute configuration
It was not possible to determine absolute configuration experimentally until 1951, when this was first achieved using X-ray crystallography with anomalous dispersion. Absolute configurations can also be determined using optical rotatory dispersion.
The concept of absolute configuration also applies to crystals of molecules with a specific chirality. See also CIP system.
- breviconic
- brewing
- Brewster angle
- Brewster, Sir David
- Brewster’s law
- Brezhnev, Leonid Ilyich (1906–82)
- BRI
- Brian Boru (c.926–1014)
- Brianchon’s theorem
- Briand, Aristide (1862–1932)
- BRIC
- brick
- brickearth
- bricks-and-clicks
- bricks and mortar company
- bricolage
- BRICS
- bridge
- bridge camera
- bridged-H network
- bridged-T network
- Bridgeman method
- bridge network
- bridge page
- bridge rectifier