The scattering of alpha particles by thin films of heavy metals, notably gold. The experiments, performed in 1909 by Geiger and Marsden under Ernest Rutherford’s direction, provided evidence for the hypothesis that atoms possessed a discrete nucleus. In Rutherford’s apparatus, a narrow beam of alpha particles from a radon source (see diagram) fell upon a thin metal foil. A glass screen coated with zinc sulphide (which scintillates on absorbing alpha particles) was placed at the end of a travelling microscope and was used to detect scattered alpha particles. The travelling microscope could be rotated about the metal foil, and by counting the number of scintillations produced in various positions during equal intervals, the angular dependence of the scattering could be determined. Since the range of alpha particles in air is limited, the central chamber of the apparatus was evacuated. The majority of alpha particles suffered only small angles of deflection (θ). However, a very small number, about 1 in 8000, were deviated by more than 90°.
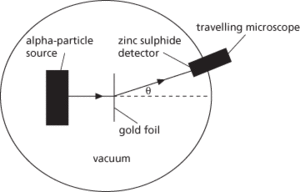
Rutherford scattering.
These findings led Rutherford to postulate that the alpha particles deflected by angles greater than 90° had encountered a small, intense positive charge of high inertia. Rutherford went on to propose in 1911 that an atom has a positively charged core or nucleus, which contains most of the mass of the atom and which is surrounded by orbiting electrons (see Bohr theory). Since very few alpha particles were scattered through large angles, it follows that the probability of a head-on collision with the nucleus is small. The nucleus therefore occupies a very small proportion of the atom’s total volume. The nucleus of an atom is of the order of 10−15 m across, whereas the atomic radius is of the order of 10−10 m, a factor of 100 000. See also nucleus.
The formula that Rutherford derived in 1911 to describe the scattering from a nucleus is known as the Rutherford scattering formula. It was derived using Newtonian mechanics but also holds in the case of quantum mechanics (except when the nucleus is helium-4; in this case the formula needs to be modified owing to the nucleus being identical to an alpha particle).
- vipassana
- viper
- VIR
- viral marketing
- Virchow, Rudolf Carl
- virga
- virgella
- Virgellina
- Virgilian
- Virginia Campaigns (July 1861–65)
- Virginius incident (1873)
- virgin medium
- Virgo
- Virgo A
- Virgo Cluster
- Virgo Supercluster
- virial coefficients
- virial equation
- virial equation of state
- virial theorem
- virion
- viroid
- virology
- Virtanen, Artturi Ilmari
- VIRTIS