A theory used in gas adsorption to describe the mass transfer of a solute from a gas into a liquid in which either side of the interface exists a stagnant gas film and a similar stagnant liquid film (see Fig. 57). The two films are assumed to offer the only resistance to mass transfer. In the bulk gas and liquid, the solution partial pressure and concentration are considered to be constant. At the interface, they are assumed to be in equilibrium. It was developed by W. K. Lewis and W. G. Whitman in 1924.
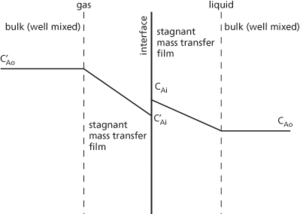
Fig. 57
- West-Kohoutek-Ikemura
- Westminster, Statute of (1931)
- Weston cell
- Weston standard cell
- WESTPAC
- Westphalia
- Westphalian
- Westphalia, Treaty of (1648)
- West Texas Intermediate
- westward drift
- West Wind Drift
- wet and dry bulb hygrometer
- wet-and-dry bulb hygrometer
- wet basis
- wet-bulb depression
- wet bulb temperature
- wet-bulb thermometer
- wet cell
- wet etching
- wet gas
- Wetherbee, James Donald (1952– )
- wetland
- wet mass
- wet melt
- wetness index