Polymers are substances that have macromolecules composed of many repeating units (known as ‘mers’). A large number of naturally occurring substances are polymers including rubber and many substances based on glucose, such as the polysaccharides cellulose and starch (in plants) and glycogen (in animals). Proteins, nucleic acids, and inorganic macromolecular substances, such as silicates, are other examples.
Synthetic polymers
One of the unique features of the chemistry of carbon is its ability to form long chains of atoms. This property is the basis of an important area of industrial chemistry concerned with the manufacture of polymeric materials with a variety of properties (see plastics). The molecules in these materials are essentially long chains of atoms of various lengths. In some polymers, cross-linkage occurs between the chains. Synthetic polymers are formed by chemical reactions in which individual molecules (monomers) join together to form larger units (see polymerization). Two types of polymer, homopolymers and heteropolymers, can be distinguished
Homopolymers
These are polymers formed from a single monomer. An example is polyethene (polyethylene), which is made by polymerization of ethene (CH2=CH2). Typically such polymers are formed by addition reactions involving unsaturated molecules. Other similar examples are polypropene (polypropylene), polystyrene, and polytetrafluoroethene (PTFE). Homopolymers may also be made by condensation reactions (as in the case of polyurethane).
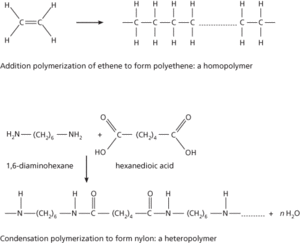
Formation of polymers
Heteropolymers
These are also known as copolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6. The reaction occurs between the amine groups on the diaminohexane and the carboxyl groups on the hexanedioic acid, with elimination of water molecules (see diagram).
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers. A well-known example is ABS (acrylonitrile-butadiene-styrene) copolymer, commonly used for the body shells of computers and other electronic apparatus. Its properties can be preselected by varying the proportions of the component monomers.
Stereospecific polymers
In both normal polyethene and nylon the polymer molecules take the form of long chains of various lengths with no regular arrangement of the subunits. Such polymers are said to be atactic. If the constituent subunits repeat along the chain in a regular way, a stereospecific polymer may result. The polymer may be isotactic, with a particular group always along the same side of the main chain, or syndiotactic, with the group alternating from side to side of the chain. Stereospecific polymerization can be performed by use of certain catalytic agents (see Ziegler process).
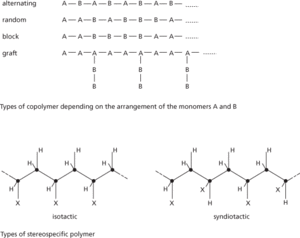
Structure of polymers
https://www.qmul.ac.uk/sbcs/iupac/BriefGuide/polymer.html Information about IUPAC nomenclature
- RNA world
- RNP
- road base
- Roadian
- road pricing
- road runner
- roadstone
- roadway construction
- Roanoke Island
- roaring forties
- roasting
- Robbins, Frederick Chapman
- Robbins, Herbert Ellis (1917–2001)
- Robert I (1274–1329)
- Robert II (1316–90)
- Robert III (1337–1406)
- Robertson, John Monteath
- Robertson, Patricia (1963–2001)
- Robertson, Sir Robert
- Robertson–Walker metric
- Roberts, Richard
- Robespierre, Maximilien François Marie Isidore de (1758–94)
- robin
- Robin boundary condition
- Robinson, Julia (Hall Bowman) (1919–85)