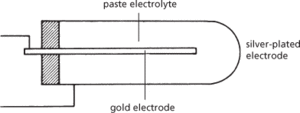
A solid-state timing device consisting essentially of a thimble-shaped electrolytic cell with a central gold electrode, an outer silver-plated electrode, and a paste electrolyte consisting of a suitable silver salt (see diagram). When a current passes, silver is lost from the outer electrode, which is made the anode, and is deposited at the same uniform rate on the central gold cathode. After a given time, determined by the elements of the external circuit, the current reverses and the cathode becomes the anode. When the cathode is ‘deplated’ the reverse current ceases and the process is once more reversed.
An E-cell is a useful and versatile timing device since it can be made very small, is robust, and can be used for a wide range of time intervals simply by altering the external circuit. The desired time interval is usually determined by charging and discharging a capacitor.
E-cell
- terrae
- terraforming
- terrain
- terrain analysis
- terrain component
- terrain correction
- terrain evaluation
- terrain pattern
- terrains of resistance
- terrain surface
- terrain unit
- terrane
- terrapin
- terra rossa
- TerraSAR-X
- terrestrial age
- Terrestrial Dynamical Time
- terrestrial magnetism
- terrestrial planet
- terrestrial radiation
- Terrestrial Time
- terric horizon
- terrier
- terrigenous
- territoriality