A reaction in which the diol pinacol, (CH3)2COH(CH3)2COH, converts to the ketone pinacolone, CH3COC(CH3)3, under acid conditions, with loss of a molecule of water. A methyl group moves from one carbon atom to an adjacent one in order to stabilize an intermediate carbocation. The reaction, also called the pinacol-pinacolone rearrangement, gives its name to a class of similar rearrangements.
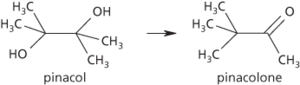
Pinacol rearrangement