See magnetism.
See magnetism.
A very weak effect that is common to all substances and is due to the orbital motion of electrons around the nucleus of atoms. Diamagnetism is independent of the temperature of the material.
If a substance is placed in a magnetic flux density B, each individual electron experiences a force due to B since it is a moving charge. The orbits and velocities of the electrons are changed in order to produce a magnetic flux density that opposes B (see electromagnetic induction). Each orbital electron therefore acquires an induced magnetic moment that is proportional to B and is in the opposite sense; hence the sample has a negative magnetic susceptibility.
Diamagnetism causes a reduction of magnetic flux density within a sample; this can be represented schematically (see diagram) by a separation of the lines of magnetic flux as they pass through the material. If a diamagnetic substance is placed in a nonuniform magnetic field it tends to move from the stronger to the weaker region of the field. A bar of diamagnetic material placed in a uniform magnetic field tends to orientate itself with the longer axis at right angles to the field. Purely diamagnetic materials include copper, bismuth, and hydrogen.
Certain materials have a permanent molecular magnetic moment and in such substances the diamagnetism is totally masked by the magnetic properties arising from this permanent moment. See paramagnetism; ferromagnetism; antiferromagnetism; ferrimagnetism.
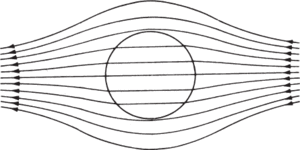
Change in magnetic flux density in a diamagnetic substance
When a magnetic field is applied to electrons orbiting a nucleus, the individual electron spins precess and result in a field in the opposite direction to that applied. All atoms and molecules show this form of magnetization, usually with a negative susceptibility of the order of 10−5 or less, but it can have paramagnetism and/or ferromagnetism superimposed upon it.
- propensity
- propensity score
- propensity score matching
- propensity to consume
- propensity to save
- propenyl group
- proper ancestor
- proper class
- proper divisor
- proper factor
- proper fraction
- proper map
- proper motion
- proper names
- proper subgraph
- proper subset
- properties
- proper time
- property
- property company
- property developer
- property diagram
- property income
- property income from abroad
- property lending