See magnetism.
See magnetism.
An effect observed in certain materials that possess a permanent atomic or molecular magnetic moment. Each orbital electron in an atom constitutes an individual current and hence has a magnetic moment; however the possible energy levels available are such that only unfilled shells of electrons contribute to the magnetic moment of the atom as a whole. The spin of the atomic electrons also has a magnetic moment associated with it but in an atom only unpaired spins contribute to the magnetic moment of the atom as a whole. Most free atoms would have a magnetic moment due to orbital electrons in unfilled outer shells but practical substances combine in general so as to complete the outer shells; most gaseous molecules and ionic or homopolar liquids or solids have no overall magnetic moment and are diamagnetic (see diamagnetism). Permanent magnetic moments are only possessed by molecules or ions containing unpaired electron spins (oxygen, O2, for example contains two unpaired spins) or by particular ions of multivalent transition elements in which there is an unfilled inner shell. Paramagnetism is most commonly associated with electron spin but a few compounds also have an orbital contribution.
In the absence of an applied magnetic flux density, thermal motion causes the individual magnetic moments to be randomly orientated throughout the sample and the net magnetization is zero. In the presence of a magnetic flux the magnetic moments tend to align themselves in the direction of the field; this tendency however is opposed by the thermal agitations and a paramagnetic substance has a small positive susceptibility, χ, which is temperature dependent.
The behaviour of a paramagnetic gas can be approximately described by the Langevin function, which shows that at ordinary magnetic fields and temperatures the gas obeys Curie’s law:
where C is a constant and T the thermodynamic temperature. However, at sufficiently high flux density and low temperature a saturation point is achieved with all the molecules aligned along the field with negligible thermal effects. Very dilute paramagnetic liquids also obey Curie’s law.
The magnetic properties of paramagnetic solids and liquids depend on the complex intra-atomic and interatomic forces operating within them and the behaviour cannot always be described by a simple equation. Nonhydrated liquids and many paramagnetic solids at ordinary temperatures and fields obey the Curie–Weiss law:
where θ, the Weiss constant, can be either positive or negative. The Curie–Weiss law is only obeyed at temperatures T > |θ| and is a modification of Curie’s law arising from the mutual interactions of the ions or molecules.
Certain metals, such as sodium or potassium, exhibit free-electron paramagnetism or Pauli paramagnetism in which a small positive susceptibility with only slight temperature dependence is observed. Both effects are due to the conduction electrons in the metals. The individual atoms in the solid are left as diamagnetic ions and the conduction electrons exhibit both diamagnetism and paramagnetism. In most metals these effects are of the same order of magnitude but Pauli paramagnetism occurs when the paramagnetism effect is greater than the diamagnetism.
At temperatures below certain critical temperatures and approximately equal to the modulus of the Weiss constant, the intermolecular forces of many paramagnetic solids become much greater than the thermal agitations. The magnetic moments are no longer randomly orientated but take up an appropriate ordered state and the materials become either ferromagnetic, antiferromagnetic, or ferrimagnetic. The paramagnetic behaviour of different materials can be compared by plotting the inverse susceptibility χ−1 against thermodynamic temperature in the paramagnetic region (Fig. a).
Paramagnetism causes an increase of magnetic flux density within a sample; this is represented schematically by a concentration of the lines of magnetic flux density passing through it (Fig. b). If a paramagnetic substance is placed in a nonuniform magnetic field it tends to move from the weaker to the stronger region of the field; a bar of paramagnetic material placed in a uniform magnetic flux tends to orientate itself with the longer axis parallel to the flux.
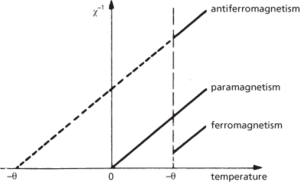
(a) Paramagnetic behaviour of different materials
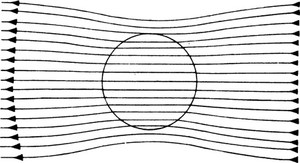
(b) Change in magnetic flux density in a paramagnetic substance
Magnetization developed in atoms or ions that have permanent intrinsic magnetic moments, in the same direction as an applied field. It is usually somewhat larger than that resulting from the diamagnetism of the material, and may have ferromagnetism superimposed upon it.
- Regional Planetary Imaging Data Facility
- regional policy
- regional registry
- regional science
- formal consequence
- formaldehyde
- formal game
- formal implication
- formalin
- formalism
- formalism in art
- formalism, legal
- formalism, logical
- formal language
- formal language theory
- formal logic
- formally, virtually, and eminently
- formal/material mode of speech
- formal method
- formal parameter
- formal sector
- formal specification
- formal system
- formal verification
- formant