A plot of neutron number (N) against proton number (Z) for nuclides belonging to a particular radioactive series. Each point marked on the plot represents a member of the series. The lines joining these points represent the nuclear transformations that have occurred in transmuting the original nucleus to the new one.
Four types of radioactive decay can be conveniently represented as shifts along a decay sequence:
Alpha decay:
The emission of an alpha particle corresponds to the loss of a helium nucleus (two protons and two neutrons) by the parent nucleus. An alpha decay is easily represented as a diagonal arrow made up of two steps down the N axis and two steps towards the left on the Z axis.
Beta (negative) decay:
A neutron in the nucleus decays into a proton and an electron; the electron is emitted as a beta particle. This transformation is therefore represented on a decay sequence as the loss of a neutron and the gain of a proton; that is, a diagonal arrow made up of one step down the N axis and one step to the right on the Z axis. See also beta decay.
Beta (positive) decay:
In this decay a proton transforms into a neutron with the emission of an antimatter electron (positron). Unlike the beta (negative) decay, which can also occur outside the nucleus, the beta (positive) decay does not occur spontaneously with free protons. This transformation is represented on a decay sequence as a diagonal arrow made up of one step up the N axis and one step to the left on the Z axis.
Electron capture:
A nuclear proton captures an electron from the atomic environment. This transformation is represented in the same way as beta (positive) decay. The diagram shows a portion of the decay sequence for the uranium series.
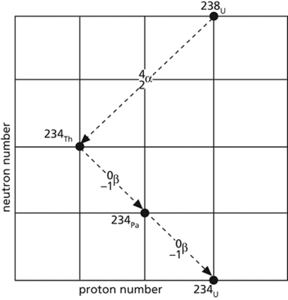
Decay sequence.
- offgassing
- office automation
- Office for Budget Responsibility
- Office for National Statistics
- Office National d'Etudes et de Recherches Aérospatiales
- Office of Communications
- Office of Fair Trading
- Office of Government Commerce
- Office of Management and Budget
- Office of Outer Space Affairs
- Office of Space Science and Applications
- Office of Telecommunications
- office software product
- Official Development Assistance
- official financing
- offlap
- off line
- offline
- off-line analysis
- offline browser
- off-page optimization
- offset
- offset QPSK
- offshore
- offshore bar