(in physical chemistry)
1. A system in which two electrodes are in contact with an electrolyte. The electrodes are metal or carbon plates or rods or, in some cases, liquid metals (e.g. mercury). In an electrolytic cell a current from an outside source is passed through the electrolyte to produce chemical change (see electrolysis). In a voltaic cell, spontaneous reactions between the electrodes and electrolyte(s) produce a potential difference between the two electrodes.
Voltaic cells can be regarded as made up of two half cells, each composed of an electrode in contact with an electrolyte. For instance, a zinc rod dipped in zinc sulphate solution is a Zn|Zn2+ half cell. In such a system zinc atoms dissolve as zinc ions, leaving a negative charge on the electrode
The solution of zinc continues until the charge build-up is sufficient to prevent further ionization. There is then a potential difference between the zinc rod and its solution. This cannot be measured directly, since measurement would involve making contact with the electrolyte, thereby introducing another half cell (see electrode potential). A rod of copper in copper sulphate solution comprises another half cell. In this case the spontaneous reaction is one in which copper ions in solution take electrons from the electrode and are deposited on the electrode as copper atoms. In this case, the copper acquires a positive charge.
The two half cells can be connected by using a porous pot for the liquid junction (as in the Daniell cell) or by using a salt bridge. The resulting cell can then supply current if the electrodes are connected through an external circuit. The cell is written
Here, E is the e.m.f. of the cell equal to the potential of the right-hand electrode minus that of the left-hand electrode for zero current. Note that ‘right’ and ‘left’ refer to the cell as written. Thus, the cell could be written
The overall reaction for the cell is
This is the direction in which the cell reaction occurs for a positive e.m.f.
The cell above is a simple example of a chemical cell; i.e. one in which the e.m.f. is produced by a chemical difference. Concentration cells are cells in which the e.m.f. is caused by a difference of concentration. This may be a difference in concentration of the electrolyte in the two half cells. Alternatively, it may be an electrode concentration difference (e.g. different concentrations of metal in an amalgam, or different pressures of gas in two gas electrodes). Cells are also classified into cells without transport (having a single electrolyte) and with transport (having a liquid junction across which ions are transferred). Various types of voltaic cell exist, used as sources of current, standards of potential, and experimental set-ups for studying electrochemical reactions. See also dry cell; primary cell; secondary cell.
2. See photoelectric cell.
3. See solar cell.
4. See Kerr effect (for Kerr cell).
See contingency table.
1. A system in which two electrodes are in contact with an electrolyte. The electrodes are metal or carbon plates or rods or, in some cases, liquid metals (e.g. mercury). In an electrolytic cell a current from an outside source is passed through the electrolyte to produce chemical change (see electrolysis). In a voltaic cell, spontaneous reactions between the electrodes and electrolyte(s) produce a potential difference between the two electrodes.
Voltaic cells can be regarded as made up of two half cells, each composed of an electrode in contact with an electrolyte. For instance, a zinc rod dipped in zinc sulphate solution is a Zn|Zn2+ half cell. In such a system zinc atoms dissolve as zinc ions, leaving a negative charge on the electrode
The solution of zinc continues until the charge build-up is sufficient to prevent further ionization. There is then a potential difference between the zinc rod and its solution. This cannot be measured directly, since measurement would involve making contact with the electrolyte, thereby introducing another half cell (see electrode potential). A rod of copper in copper sulphate solution comprises another half cell. In this case the spontaneous reaction is one in which copper ions in solution take electrons from the electrode and are deposited on the electrode as copper atoms. In this case, the copper acquires a positive charge.
The two half cells can be connected by using a porous pot for the liquid junction (as in the Daniell cell) or by using a salt bridge. The resulting cell can then supply current if the electrodes are connected through an external circuit. The cell is written
Here, E is the e.m.f. of the cell equal to the potential of the right-hand electrode minus that of the left-hand electrode for zero current. Note that ‘right’ and ‘left’ refer to the cell as written. Thus, the cell could be written
The overall reaction for the cell is
This is the direction in which the cell reaction occurs for a positive e.m.f.
The cell above is a simple example of a chemical cell; i.e. one in which the e.m.f. is produced by a chemical difference. Concentration cells are cells in which the e.m.f. is caused by a difference of concentration. This may be a difference in concentration of the electrolyte in the two half cells. Alternatively, it may be an electrode concentration difference (e.g. different concentrations of metal in an amalgam, or different pressures of gas in two gas electrodes). Cells are also classified into cells without transport (having a single electrolyte) and with transport (having a liquid junction across which ions are transferred). Various types of voltaic cell exist, used as sources of current, standards of potential, and experimental set-ups for studying electrochemical reactions. See also dry cell; primary cell; secondary cell; lithium battery.
2. See Kerr effect (for Kerr cell).
1. A simple living organism such as a bacterium or yeast. Bacteria reproduce by binary division whereas yeast cells reproduce through budding. They are used as biocatalysts in biochemical reactions such as fermentation, converting sugars into alcohol and carbon dioxide and used in the food and brewing industries.
2. A sealed chamber used in the nuclear reprocessing industry in which a process takes place. There is no access by process operators during normal operation. Control of the process is therefore carried out remotely.
3. A system in which two electrodes are in contact with an electrolyte. In a voltaic cell, electrochemical energy is produced by a chemical reaction that takes place between two electrodes made from different metals in an electrolyte consisting of salts or acidic substances. A voltaic cell is also known as a galvanic cell. See battery.
1. An address, a location in memory, or a register, usually one capable of holding a binary number. It is sometimes a location capable of holding one bit.
2. The basic unit of a spreadsheet or some other table of text, formed by the intersection of a row and column. It contains a label, value, or formula with attributes such as size, font, and colour.
3. The name given to a packet in one version of a packet switching system. Packet switching systems subdivide the data to be transmitted into a number of packets. In contrast to many systems, a cell is short—for instance 53 bytes in the case of an ATM cell—and its internal structure is fixed. Small size and fixed structure allow the cell to be switched using a very simple algorithm; the processing time required for switching is thus reduced, with a corresponding increase in the number of cells switched in a given time.
4. The coverage area provided by a base station to a mobile (wireless) phone. As the user moves geographically, the conversation is ‘handed over’ to another cell at another base station.
1. (electrolytic cell; voltaic cell) A device that produces electricity by chemical means, consisting of a pair of plate electrodes in an electrolyte. In a primary cell the chemical action is not normally reversible, the current being produced as a result of the dissolution of one of the plates. A secondary cell has reversible chemical action and is charged by passing a current through it. The rate and direction of the chemical action is determined by the value of the external voltage. The volt efficiency of a secondary cell is the ratio of the voltage developed by it during the discharge to the average voltage supplied to it during the recharging cycle.
The cell internal resistance is the resistance offered to the passage of current inside the cell. If the open-circuit e.m.f. is E, then the potential difference, U, across the cell when current flows is given by
where R is the external resistance and r the internal resistance.
A portable cell that has the electrolyte in the form of a nonspillable jelly or paste is known as a dry cell. Most cells contain a liquid electrolyte and are sometimes termed wet cells. Dry cells are used in the batteries for torches, portable radios, etc.
2. Any device that can generate a direct electromotive force from a nonelectrical source of energy, particularly from light energy. Examples include solar cells and photovoltaic cells.
The structural and functional unit of most living organisms (compare coenocyte; syncytium). Cell size varies, but most cells are microscopic (average diameter 0.01–0.1 mm). Cells may exist as independent units of life, as in bacteria and certain protists, or they may form colonies or tissues, as in all plants and animals. Each is differentiated into cytoplasm and a nucleus, which contains DNA, and is bounded by a plasma membrane, which in the cells of plants, fungi, algae, and bacteria is surrounded by a cell wall. There are two main types of cell. In prokaryotic cells (bacteria and archaea) the nuclear material is not bounded by a membrane and chemicals involved in cell metabolism are associated with the plasma membrane. Reproduction is generally asexual and involves simple cell cleavage. In eukaryotic cells the nucleus is bounded by a nuclear membrane and the cytoplasm is divided by membranes into a system of interconnected cavities and separate compartments (organelles), e.g. mitochondria, endoplasmic reticulum, Golgi apparatus, lysosomes, and ribosomes (see illustration). The shape and internal organization of cells depends on a network of tubules and filaments called the cytoskeleton. Reproduction can be either asexual (see mitosis) or sexual (see meiosis). Plants and animals consist of eukaryotic cells but plant cells possess chloroplasts and other plastids and bear a rigid cellulose cell wall.
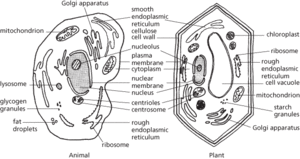
Generalized eukaryotic cells
https://www.cellsalive.com/toc_cellbio.htm Overview of cell biology, with animations and illustrations, from Cells Alive!
- orogenic belt
- orogenic cycle
- orogeny
- orographic
- orographic precipitation
- OROM
- OR operation
- Orosirian
- orphan link
- orphan page
- orphan receptor
- Orphism
- orpiment
- orrery
- Orsat analysis
- Orsini, Felice (1819–58)
- Ortega, Daniel
- Ortega y Gasset, José (1883–1955)
- Orthida
- orthids
- orthite
- ortho-
- orthoamphiboles
- orthoboric acid
- orthocentre