1. Organic compounds containing the group –CO.NH2 (the amide group). Compounds containing this group are primary amides. Secondary and tertiary amides can also exist, in which the hydrogen atoms on the nitrogen are replaced by one or two other organic groups respectively. Simple examples of primary amides are ethanamide, CH3CONH2, and propanamide, C2H5CONH2. They are made by heating the ammonium salt of the corresponding carboxylic acid. Amides can also be made by reaction of ammonia (or an amine) with an acyl halide. See also Hofmann’s reaction.
2. Inorganic compounds containing the ion NH2-, e.g. KNH2 and Cd(NH2)2. They are formed by the reaction of ammonia with electropositive metals.
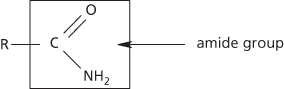
Amides
https://www.acdlabs.com/iupac/nomenclature/93/r93_543.htm Information about IUPAC nomenclature
- Ajdukiewicz, Kazimierz (1890–1963)
- Ajne test
- AKA
- Akaike, Hirotugu (1927–2009)
- Akaike's information criterion
- Akari
- Akatsuki
- Akbar, Jalaludin Muhammad (1542–1605)
- Akebono
- Akers, Thomas Dale (1951– )
- Akhenaten (14th century bc)
- akinete
- Akiyama, Toyohiro (1942– )
- Akkad
- aklé
- akolasia
- akoluthic
- akrasia
- Aksayan
- Aksum
- aktuopalaeontology
- al-
- alabaster
- Alamein, El, Battle of (October–November 1942)
- Alamo, the