The existence of atomic nuclei that have the same atomic number and the same mass number but different energy states.
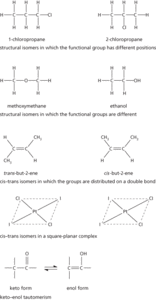
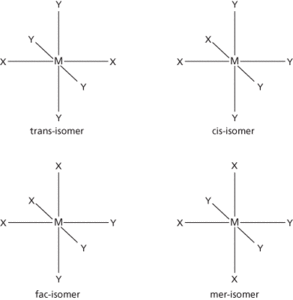
The existence of chemical compounds (isomers) that have the same molecular formulae but different molecular structures or different arrangements of atoms in space. In structural (or constitutional isomerism) isomerism the molecules have different molecular structures: i.e. they may be different types of compound or they may simply differ in the position of the functional group in the molecule. Structural isomers generally have different physical and chemical properties. In stereoisomerism, the isomers have the same formula and functional groups, but differ in the arrangement of groups in space. Optical isomerism is one form of this (see optical activity). Another type is cis-trans isomerism (formerly geometrical isomerism), in which the isomers have different positions of groups with respect to a double bond or ring or central atom. Octahedral complexes can display cis-trans isomerism if they have formulae of the type MX2Y4. Octahedral complexes with formulae of the type MX3Y3 can display a different type of isomerism. If the three X ligands are in a plane that includes the metal atom and the three Y ligands are in a different plane at right angles, then the structure is a mer-isomer (meridional). If the three X ligands are all on one face of the octahedron and the three Y ligands are on an opposite face, then it is a fac-isomer (facial). See also ambidentate; e–z convention.
Isomerism
Isomerism
The existence of two or more substances having the same chemical composition but different arrangements of their atoms e.g. butane (C4H10) has two isomers: as a straight four-carbon chain and as a three-carbon chain with a methyl group (−CH3) in the middle. There are two types of isomerism: structural (i.e. butane) and stereoisomerism, which includes optical and geometric isomerism.
- CRL
- CRM
- CRM system
- CRO
- Croatia
- Croce, Benedetto (1866–1952)
- crocidolite
- Crockett, David (1786–1836)
- Crocodilia
- Croesus (560–546)
- Croixian
- Croll, James
- Cromerian
- Crommelin
- Crommelin, Andrew Claude de la Cherois (1865–1939)
- Cromwell current
- Cromwell, Oliver (1599–1658)
- Cromwell, Richard (1626–1712)
- Cromwell, Thomas (1485–1540)
- Cronbach, Lee Joseph (1916–2001)
- Cronbach's alpha
- Cronin, James Watson
- cronstedite
- Crookes dark space
- Crookes, Sir William