Compounds that react with water to form an acid. For example, carbon dioxide reacts with water to give carbonic acid:
A particular group of acid anhydrides are anhydrides of carboxylic acids. They have a general formula of the type R.CO.O.CO.R′, where R and R′ are alkyl or aryl groups. For example, the compound ethanoic anhydride (CH3.CO.O.CO.CH3) is the acid anhydride of ethanoic (acetic) acid. Organic acid anhydrides can be produced by dehydrating acids (or mixtures of acids). They are usually made by reacting an acyl halide with the sodium salt of the acid. They react readily with water, alcohols, phenols, and amines and are used in acylation reactions.
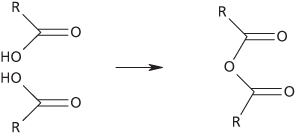
Acid anhydride
https://www.acdlabs.com/iupac/nomenclature/93/r93_534.htm Information about IUPAC nomenclature