Shapes that can be taken by nonplanar rings and can be interconverted by formal rotations about single bonds. In cyclohexane, for example, the most stable conformation is the chair conformation in which the 2, 3, 5 and 6 atoms lie in a plane and the 1 and 4 carbon atoms lie on opposite sides of the plane. In this form, there is no ring strain. In the boat conformation, the 1 and 4 carbon atoms lie on the same side of the plane. Other conformers are the twist conformation and the half chair conformation (see diagram). The energy difference between the chair conformation and the (least stable) half chair is 10.8 kcal/mol and the forms interconvert quickly at normal temperatures (about 99% of molecules are in the chair form).
Other rings also have different conformations. In a five-membered ring, such as that of cyclopentane, the twist conformation has three adjacent carbon atoms in a plane with one of the carbons above this plane and the other below it. The less stable envelope conformation has four atoms in a plane with one atom out of the plane. In an eight-membered ring, such as that of cyclooctane, the tub conformation has four atoms corresponding to a pair of diametrically opposite bonds in one plane, with the other four atoms on the same side of this plane. It is analogous to the boat form of cyclohexane. The crown conformation has atoms alternatively above and below the average plane of the molecule.
Various names are given to positions attached to atoms in different ring conformations. In the chair conformation of cyclohexane, an axial bond is one that makes a large angle with the average plane of the ring. An equatorial bond is one that makes a small angle. In a cyclopentane ring, the terms pseudo-axial and pseudo-equatorial are used. In the boat conformation of cyclohexane the bond roughly parallel to the plane of four atoms is the bowsprit, the other bond being the flagpole.
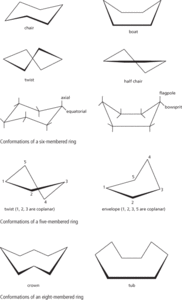
Ring conformations
- annealing
- annelation
- Annelida
- Anne of Austria (1601–66)
- Anne of Cleves (1515–57)
- Anne, St
- Anniceris (320–280)
- annihilation
- annihilator
- Anning, Mary (1799–1847)
- annotation
- annotations((in bioinformatics))
- announcement effect
- announcement newsgroup
- announcement of opportunity
- annoyance bot
- annual
- annual aberration
- annual equation
- annual general meeting
- annual inequality
- annualized growth rate
- annualized percentage rate of interest
- annual parallax
- Annual Population Survey