The formation of images to provide information about the function of various organs in the body, using internally administered radioisotopes as a radiation source. The technique is widely used in medicine to locate tumours or cancers and to examine the flow patterns of body fluids.
Alpha and beta radiation is not suitable for imaging procedures as they are both absorbed by tissue, subjecting healthy cells to an unnecessary dose of radiation. Gamma radiation, however, is not easily absorbed by tissue and can therefore be conveniently detected outside the body. The gamma radiation emitted from the area under examination is detected either by a rectilinear scanner or a gamma camera.
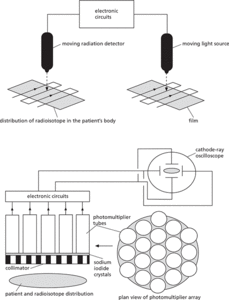
In the rectilinear scanner, the radiation detector is mounted onto an armature that moves in straight lines as shown in the diagram. The intensity of the radiation from the radioisotope within the patient’s body is picked up and transformed by electronics into a signal that controls the intensity of light produced by a light source. The light source mimics the movement of the detector exactly, enabling an image of the distribution of the radioisotope to be produced.
In the gamma camera, the gamma radiation emitted from the radioisotope distributed in the patient’s body is detected by a series of sodium iodide crystals set behind a collimator. The sodium iodide crystals produce a flash for each detected gamma ray. The light from the flash is amplified in photomultiplier tubes, which are arranged in an array to enable different tubes to correspond to different regions of the body under examination. Electronic circuits convert the output of the photomultiplier array into electrical potential differences, which are applied across the X and Y plates of a cathode-ray oscilloscope to form a two-dimensional image of the distribution of the radioisotope.
Nuclides are often injected into the patient in the form of a dissolved compound, or sometimes attached to antibodies. Pure gamma-emitters of energy 60–40 keV are usually chosen as radioisotopes for these procedures. The nuclide should also have a suitable half-life, typically a few hours, so that the overall radiation dose is kept to a minimum. However, the activity must remain high enough for a clinical examination to take place. It is also important that the nuclide and its carrier fluid are nontoxic and sterile. Two isotopes commonly used for this procedure are iodine–131 (131I) and technecium–99 (99Tc). 131I is produced in a nuclear reactor, when neutrons are captured by tellurium (13052Te). The isotope formed then decays by the emission of a beta particle into 131I. The iodine is then separated chemically from the tellurium:
131I has a half-life of 8 days, and itself decays by beta emission, enabling it to be sometimes used to treat thyroid cancer (see radiology). However, it also emits a gamma ray of suitable energy for detection (360 keV):
The 8-day half-life is ideal for clinical purposes. 131I is commonly used for thyroid studies, since the thyroid gland readily takes up iodine from the bloodstream. 99Tc used in the imaging procedure is a metastable state; that is, it is a nuclide remaining in an excited state over a long half-life. The metastable 99Tc (usually labelled 99Tc*) decays to a ground state by gamma emission. The 99Tc* itself is a product of the beta decay of a molybdenum radionuclide:
The second process in this chain has a half-life of 6 hours, which is ideal for relatively short clinical studies. The gamma ray emission is also at an energy of 160 keV, which is easily detectable. No harmful beta particles are produced.
99Tc* is obtained from a device, sometimes called a ‘Mo-cow’ or ‘molybdenum cow’, on account of the way in which the isotope is produced and tapped off the apparatus. The device operates by elution of a specially prepared column of ammonium molybdenate. The molybdenum in this column is originally obtained from the fission of uranium in a nuclear reactor. The metastable technetium occurs in the column in the form of a pertechnate ion, which can be exchanged with chloride ions when sodium chloride solution is passed through the column. In this way pertechnate ions are removed from the column, leaving behind the molybdate that has not yet decayed. The fluid that one ‘milks’ from this apparatus is sterile and compatible with blood. 99Tc* has many applications in imaging the brain or tumours, which have rich or poor blood supplies.
Radioisotope imaging.
- class equation
- class F amplifier
- class frequency
- class G amplifier
- class H amplifier
- classical Cepheid
- classical control
- classical dichotomy
- classical economics
- classical electrodynamics
- classical field theory
- classical logic
- classical mechanics
- classical model
- classical negation
- classical physics
- classical unemployment
- classicism
- classification
- Classification Theorem for Surfaces
- classification tree
- classifier systems
- class interval
- Classless Inter-Domain Routing
- class limit